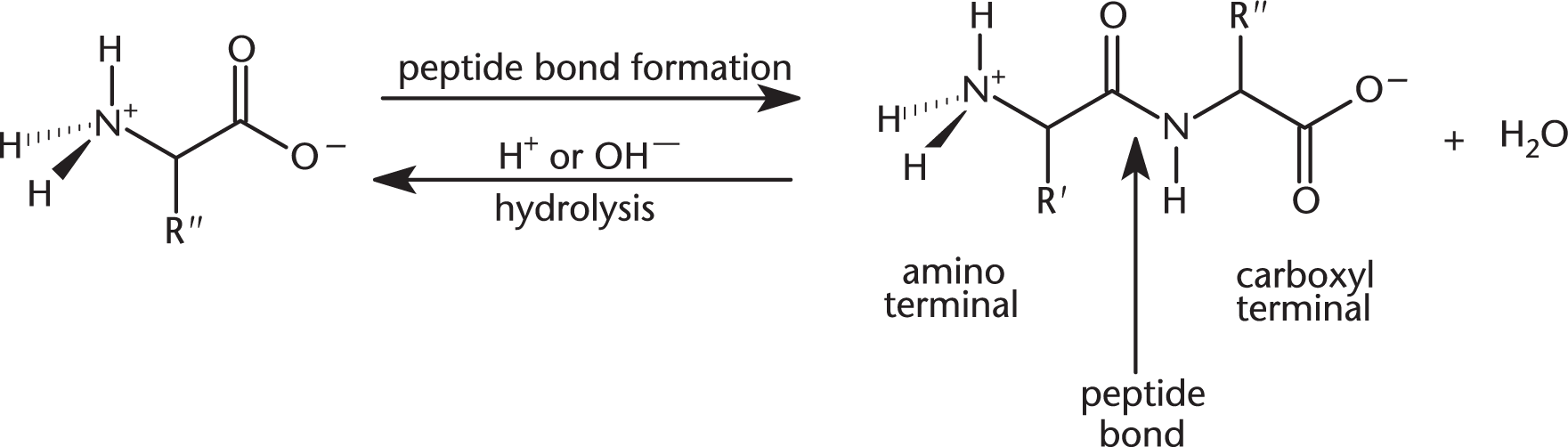
Peptides are composed of amino acid subunits, sometimes called residues, linked by peptide bonds. Two amino acids joined together form a dipeptide, three form a tripeptide, and many amino acids linked together form a polypeptide.
Amino acids are joined by peptide bonds (amide bonds) between the carboxyl group of one amino acid and the amino group of another. This bond is formed via a condensation reaction (a reaction in which water is lost). The reverse reaction, hydrolysis (cleavage with the addition of water) of the peptide bond, is catalyzed by an acid or base.
Certain enzymes digest the chain at specific peptide linkages. For example, trypsin cleaves at the carboxyl end of arginine and lysine; chymotrypsin cleaves at the carboxyl end of phenylalanine, tyrosine, and tryptophan.

The terminal amino acid with a free alpha-amino group is known as the amino-terminal or N-terminal residue, while the terminal residue with a free carboxyl group is called the carboxy-terminal or C-terminal residue. By convention, peptides are drawn with the N-terminal end on the left and the C-terminal end on the right.
Amides have two resonance structures, and the true structure is a hybrid with partial double-bond character. As a result, rotation about the C−N bond is restricted. The bonds on either side of the peptide unit, however, have a great deal of rotational freedom.
