
Structural lipids are a major component of the phospholipid bilayer. The structure of phospholipids—hydrophilic head and hydrophobic tail—allows for bilayer formation and protects the cell from the surrounding environment. Phospholipids are amphipathic, meaning they have both hydrophilic and hydrophobic regions. This allows the formation of other structures in addition to the phospholipid bilayer, such as micelles and liposomes.

Phospholipids have a polar hydrophilic head (made up of a phosphate and an alcohol) joined to a hydrophobic fatty acid tail by phosphodiester linkages. The number of fatty acid tails attached to the polar head can vary according to the type of phospholipid. Different molecules also provide the structure upon which the phospholipid is built. For example, glycerol, which is a three-carbon alcohol molecule, makes up phosphoglycerides and glycerophospholipids, whereas sphingolipids have a sphingosine backbone.
All lipids have a fatty acid tail. This chain can vary in both saturation and length. Fully saturated fatty acid tails have only single bonds (therefore, each carbon is bonded to four other atoms). These fatty acids are more stable and form solids at room temperature; these are the fatty acids found in butter. Unsaturated fatty acids have one or more double bonds in their structure, which create kinks in the fatty acid chains. This keeps the chains from stacking on top of each other and solidifying; therefore, these fats tend to be liquid at room temperature (e.g., olive oil). This is also why the phospholipid bilayer acts fluidly at body temperature.
Most double bonds in fatty acids are in the cis- configuration. Trans- double bonds, often referred to as trans-fatty acids, manifest when partially hydrogenated vegetable oils are used in production. These fatty acids decrease membrane fluidity and, along with saturated fatty acids, are associated with increased risk of atherosclerosis.
Glycerophospholipids are a type of phospholipid that contains a glycerol backbone. Depending on the type of glycerol in the head group, the name of the phospholipid varies. These head groups can be positively or negatively charged or they can be neutral. This polarity can dictate the glycerophospholipid’s role in cell recognition, signaling, binding, and so forth. As in all phospholipids, the fatty acid chains can vary in length and saturation, introducing another variation in the lipid’s structure. Glycerophospholipids are important components in membrane synthesis.
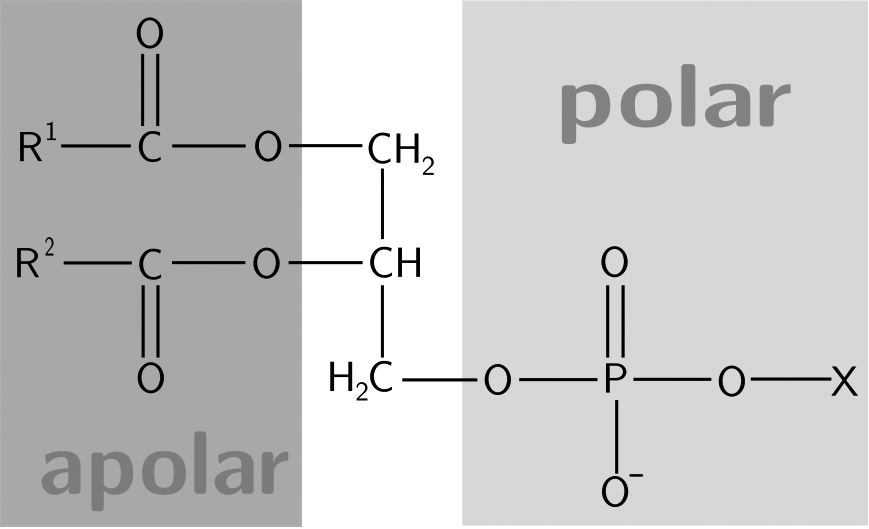
Sphingolipids have a sphingosine or sphingoid backbone and have long, nonpolar fatty acid tails and polar head groups as in other phospholipids. An example of a sphingolipid is the antigens on the surface of red blood cells that form the basis for ABO blood typing.
Waxes consist of esters of long chain fatty acids with long chain alcohols. They form malleable solids at room temperature and function biologically as protection against the environment. In humans, waxes help prevent dehydration and provide lubrication.