![]()
![]()
![]()
![]()
Genetic Counseling and Testing
![]()
![]()
![]()
![]()
![]()
![]()
![]()
Breast cancer was recognized in families as far back as Roman times. In the mid-1800s, a famous French surgeon described multiple cases of breast cancer occurring in his own family.
In the modern era, there were two main factors that unlocked the centuries-old puzzle of breast cancer occurring in families. First — in the latter half of the 20th century — was the careful collection of family histories and blood samples from family members with a recognized breast cancer link. Second has been the development of genomic technology that can be used to study these breast cancer families. This has occurred in the past 25 years, with many more developments expected in the future.
As we begin this chapter, it’s important to understand there’s a difference between having some family history of breast cancer (such as one or two relatives) versus a clear hereditary cancer pattern. In the pages that follow, we describe these differences in greater detail. Once you better understand the genetics of breast cancer and how genetic testing works, you can read about the options for reducing breast cancer risk, explained in Chapter 6.
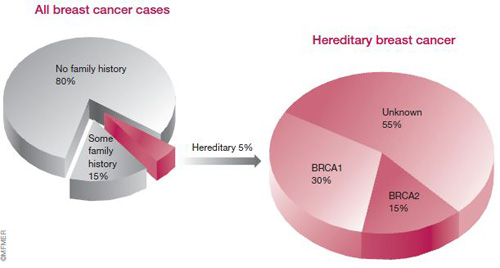
When a woman is diagnosed with breast cancer, one of the first things her doctor wants to determine is if she has a hereditary form of the disease. That is, is her cancer the result of an abnormal gene that’s being passed on in the family?
Gathering a detailed family health history is an important first step in identifying a potential hereditary cancer. Clues that point to inherited breast cancer include:
Nonhereditary disease
Just because a woman has one or two relatives with breast cancer doesn’t mean there’s a strong inherited abnormality in the family. In most women with a family history of breast cancer, there isn’t one specific inherited gene that’s responsible for the cancer. Instead, multiple factors that increase breast cancer risk are likely at play.
Breast cancer may occur more often in some families because of shared reproductive or lifestyle risk factors. In these women, their risk of breast cancer is much lower than that of women with an inherited mutation. For a woman who has a mother or sister with breast cancer and no other affected relatives and no identified genetic alteration, the probability that she will develop breast cancer by age 70 is between 7 and 18 percent. The risk increases as the number of relatives with breast cancer goes up, but it is still less than the risk for women who carry a known genetic mutation.
Hereditary disease
About 5 to 10 percent of all breast cancers are thought to be hereditary — caused by an inherited alteration (mutation) in a single gene. Several of these genes, known as cancer susceptibility genes, have been identified.
The first genes to be discovered were breast cancer gene 1 (BRCA1) and breast cancer gene 2 (BRCA2). Together, defects in these genes account for about 45 percent of hereditary breast cancer cases, or about 1.5 to 3 percent of all breast cancers.
BRCA1
Defects in the BRCA1 gene, located on chromosome 17, appear to be responsible for breast cancer in about 30 percent of families with multiple cases of the disease. They’re also responsible for breast cancer in the large majority of families who have both breast and ovarian cancers. By age 70, a woman who carries a mutation in this gene has about a 54 percent chance of developing breast cancer and a 39 percent chance of getting ovarian cancer.
BRCA1 and BRCA2 carriers who’ve been diagnosed with breast cancer also have a higher risk of developing a second cancer in the opposite breast. The level of risk depends on a woman’s age when receiving her first breast cancer diagnosis — as her age increases her risk decreases. A woman who was younger than age 40 when she developed her first breast cancer has about a 60 percent chance of developing breast cancer in the opposite breast over the next 25 years.
Defects in the BRCA1 gene may also be associated with an increased risk of fallopian tube and primary peritoneal cancer (See Chapter 17 for more information about this risk and what you can do.) Men with a BRCA1 mutation have a threefold increased risk of prostate cancer.
BRCA2
Mutations in the BRCA2 gene, located on chromosome 13, account for about 15 percent of hereditary breast cancers. By age 70, carriers of a BRCA2 mutation have about a 45 percent chance of developing breast cancer and a 16 percent chance of developing ovarian cancer.
BRCA2 mutations may also be associated with an increased risk of several other cancers, including cancers of the prostate, pancreas, gallbladder and stomach, as well as the skin cancer melanoma. BRCA2 families also have an increased risk of male breast cancer.
≈≈≈≈≈≈≈
For years, researchers had known that women with a strong family history of breast cancer were at higher risk of getting the disease than were other women. In 1994, they identified the first gene with a clear association to hereditary breast cancer — BRCA1.
The search for a gene responsible for breast cancer began with studies of families with multiple cases of breast cancer. By studying these families, researchers were able to identify a pattern (autosomal dominant inheritance pattern) for how the disease was passed on to family members (see Passing on a defective gene).
With the advent of technology that made it possible to analyze DNA, researchers were able to begin searching for the actual gene that was altered in people with a family history of breast cancer. In 1990, a team of scientists narrowed the search by discovering a link between early-onset breast cancer in families and a region of chromosome 17. The researchers called the region BRCA1. Four years later, the region was narrowed to the specific gene where mutations were found to occur. This discovery enabled the development of genetic testing for mutations in BRCA1. The first commercial testing for BRCA1 mutations was performed in 1996.
BRCA2 was identified on chromosome 13 in 1995.
Discovery of BRCA1

Data from Miki Y, et al. Science, 1994;266:66 and other sources.
≈≈≈≈≈≈≈
The role of BRCA genes
BRCA1 and BRCA2 are tumor suppressor genes found in all humans. Normally, they regulate activity within a cell that helps to suppress the chance of cancer development. The BRCA genes produce proteins that help detect and repair DNA damage that can occur during normal cell division. When a BRCA gene is altered (mutated), the DNA repair process can go awry, and genetic defects can accumulate. This allows abnormal cells to multiply and cancer to develop.
BRCA1 and BRCA2 are both very large genes that contain codes for large proteins. More than 1,500 distinct mutations in BRCA1 and BRCA2 have been described. For example, in one mutation seen in women of Ashkenazi Jewish ancestry, just two pieces of the genetic code are missing out of a sequence of 6,000 — this one small deletion can result in an increased susceptibility to breast cancer. The mutations associated with an increased risk of cancer cause either missing or nonfunctional protein products.
Breast tumors that arise as a result of an inherited BRCA1 defect have some features of more aggressive cancers — their cells appear more abnormal (higher grade), and they usually are estrogen receptor negative. These traits are explained in Chapter 8. BRCA2-related breast cancers are more likely to be estrogen receptor positive, similar to nonhereditary breast cancers. Estrogen receptor status is one of many factors used to determine the best course of treatment.
BRCA in a nutshell
Before BRCA1 and BRCA2 were found, women with a strong family history of breast cancer were usually assumed to be carriers of a gene mutation, but no tool was available for testing these women. Thanks to advances in gene mapping and genetic testing, many women can now be tested for genetic mutations to help guide them in making decisions.
It’s important to note that in families where an altered BRCA1 or BRCA2 gene is present, not all family members will inherit the gene mutation. That’s why genetic testing may be especially helpful. Only family members who test positive for a known genetic mutation are at increased risk. In family members who aren’t carriers, their risk isn’t any greater than is the average woman’s, and they won’t pass the gene on to their children.
It’s also important to understand that BRCA1 and BRCA2 account for only a portion of hereditary breast cancers (see the figure earlier this chapter). Researchers continue to work to identify other breast cancer susceptibility genes. More specifics on genetic testing can be found later in this chapter.

In this example of a BRCA1 family, the mother has one normal and one abnormal BRCA1 gene. That means, each child has a 50-50 chance of inheriting the abnormal gene, which predisposes the child to increased cancer risk. The couple shown here has four children, a son and three daughters. Each child gets one copy of BRCA1 from the mother and one from the father. The mother’s mutation is passed on to two of her children, including her son. He doesn’t develop breast cancer, but he passes the mutation on to one of his daughters.
Mutations in BRCA1 or BRCA2 are inherited in what’s called an autosomal dominant pattern. What this means is that one parent, either the father or the mother, has one normal copy of the gene and one abnormal copy. Either gene can be passed on. That means, each child has a 50 percent chance of inheriting the mutation.
Because both men and women have chromosomes 17 and 13, a mutation in one of these genes can come from either your father or your mother. Women may not realize they have a family history of breast cancer if the cancer is on their father’s side of the family. Inheriting a genetic mutation associated with the development of breast cancer greatly increases the likelihood of getting the disease, but it’s not a given. Some women with a BRCA1 or BRCA2 mutation develop early and multiple cancers, while others develop cancer later in life or not at all.
There are several possible reasons for the individual variation in cancer susceptibility among carriers of BRCA1 or BRCA2 alterations. First, genetic mutations located at different places in these genes may lead to different levels of risk. Second, other risk factors, such as a woman’s reproductive history, may influence breast cancer risk associated with these mutations. Lifestyle factors may also interact with the genetic susceptibility, and other genes may interact with the BRCA genes to modify an individual’s risk.
Estimated breast cancer risk in BRCA mutation carriers
| Age | BRCA1 carriers | BRCA2 carriers |
| 30 | 1.8% | 1% |
| 40 | 12% | 7.5% |
| 50 | 29% | 21% |
| 60 | 44% | 35% |
| 70 | 54% | 45% |
Estimated ovarian cancer risk in BRCA mutation carriers
| Age | BRCA1 carriers | BRCA2 carriers |
| 30 | 1% | 0.2% |
| 40 | 3.2% | 0.7% |
| 50 | 9.5% | 2.6% |
| 60 | 23% | 7.5% |
| 70 | 39% | 16% |
This chart indicates the likelihood, by age, that a 20-year-old carrier of a mutant BRCA gene will develop ovarian cancer. For example, by age 70, 39 out of 100 BRCA1 carriers are expected to develop ovarian cancer.
Data from Sining Chen and Giovanni Parmigiani, Meta-Analysis of BRCA1 and BRCA2 Penetrance. Journal of Clinical Oncology, 2007;25:1329.
Women thought to be at high risk of carrying an inherited genetic mutation for breast cancer are often referred to a geneticist for a cancer risk assessment. A geneticist is a medical professional who specializes in genes and heredity. A genetic assessment may include genetic counseling and testing.
For breast cancer and other women’s cancers, such as ovarian cancer, genetic testing analyzes one or more specific genes — usually BRCA1 and BRCA2. Genetic testing can help determine whether you carry a specific genetic mutation that puts you at increased risk of cancer. And it can let you know what is the probability that you’ll develop the disease in your lifetime.
Genetic testing is a valuable tool that can be used to help doctors gauge a woman’s breast cancer risk. But remember that it’s just one component of a comprehensive cancer risk assessment plan.
Who should be tested?
Doctors generally recommend genetic testing only for individuals with a family history that suggests they may be carriers of a BRCA1 or BRCA2 mutation.
The preferred approach is to begin the testing in a family member who has developed breast or ovarian cancer, since that person is the most likely to have a gene mutation. If a mutation is found in this person, then other relatives can be tested for the same mutation.
Family members who are found to have the same mutation are gene carriers with increased cancer risks. An individual who tests negative for the mutation has the same risk level as women in the general population.
If it’s not possible to begin testing in a family member who has had breast or ovarian cancer, then a person without cancer can be tested. If the test reveals a mutation that’s known to increase breast cancer risk in other families, then the person is a gene carrier with an elevated cancer risk.
If no mutation is found, there are a couple of possible explanations. It may be that there is a BRCA1 or BRCA2 mutation present in this family, but it happens that the individual tested is negative for it — she or he is not a carrier. Another possibility is that BRCA1 or BRCA2 mutations are not present in this family, but another mutation in some other gene is present — one that we cannot test for at this time (a so-called BRCA-negative family). In this case, the person may or may not be a carrier.
Obviously, the risk associated with these different scenarios is very different, underscoring the need to work with a genetic counselor as testing is done.
≈≈≈≈≈≈≈
To help determine if you have an inherited risk of breast cancer, your doctor may construct your pedigree. A pedigree is a chart that shows your family tree and indicates which family members developed cancer, what kind of cancer they had and at what age they developed it. A pedigree, like the one illustrated below, can reveal a strong inheritance pattern for breast, ovarian and other cancers.
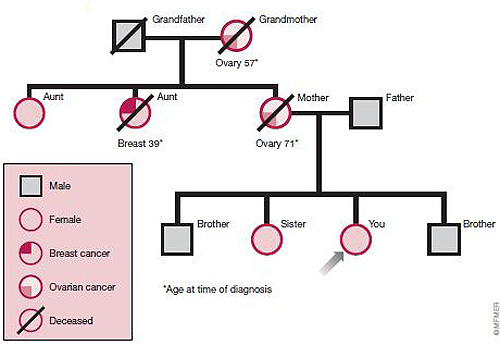
≈≈≈≈≈≈≈
If you are referred for genetic counseling, you may have many questions. Feel free to ask about any concerns you may have. They may include:
≈≈≈≈≈≈≈
BRCA-negative families
In many families with a hereditary pattern for breast cancer, BRCA1 and BRCA2 testing is done and the results come back negative. In these families, there’s likely a gene at work responsible for the cancer, but we don’t yet know the identity of that particular gene. In these situations, women who develop breast cancer are thought to be carriers of the gene mutation. Family members who don’t develop breast cancer may or may not be carriers.
In more than half of inherited breast cancers, the specific genes involved are unknown (see figure earlier this chapter). An example of a BRCA-negative family is highlighted in this chapter.
Even when BRCA1 and BRCA2 test results come back negative, genetic counseling is helpful. As new genes that predispose women to breast cancer are identified, tests for the genes are developed. A genetic counselor can also help with options for reducing cancer risk.
What’s involved?
Genetic counseling is typically recommended to make sure you fully understand the risks, benefits and psychological effect of learning that you may carry a gene that puts you at increased risk of developing cancer. The counseling team may include a genetic counselor, nurse, doctor, psychologist and social worker.
A major topic covered during a genetic consultation is the likelihood that you have inherited a BRCA1 or BRCA2 mutation. The tables below show the likelihood of a person inheriting a mutation in BRCA1 or BRCA2. The estimate is based on the individual’s family history of cancer (shown across the top) and the individual’s own cancer history (shown down the far left column).
For example, in the table below, Beth has been diagnosed with ovarian cancer. She has no family history of ovarian cancer, but her cousin was diagnosed with breast cancer at age 45. These combined factors, as is shown in the table, put Beth’s chance of having a BRCA1 or BRCA2 mutation at 14.3 percent. Meanwhile, Beth’s sister Carol has not had cancer. The chance that Carol has inherited a mutation is only 2.6 percent.
As you look at these tables, you see the features that significantly increase a person’s chance of having a mutation, such as ovarian cancer in the family. The table below applies to non-Ashkenazi individuals. Because BRCA1 and BRCA2 mutations are more common in individuals of Ashkenazi Jewish descent, a separate table is necessary for them (see table below). Again, the table shows the likelihood that a person has inherited a mutation, based on that person’s family history and personal cancer history. Please keep in mind that the figures in these tables are only estimates and should not be taken as guarantees.
Other topics covered during a genetic consultation may include:
Estimated likelihood of having a BRCA1 or BRCA2 mutation
(Non-Ashkenazi population)
Family History
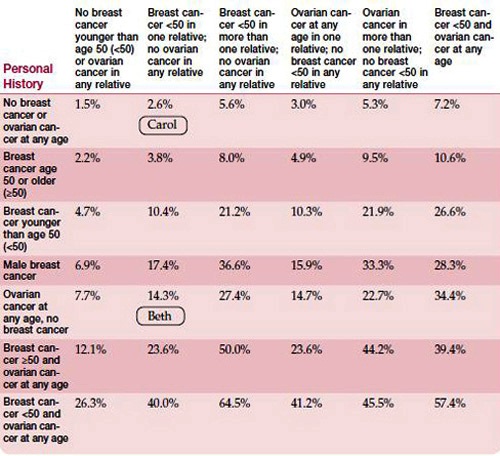
Adapted from BRCA1 and BRCA2 Prevalence Tables. Myriad Genetic Laboratories, Inc.
Estimated likelihood of having a BRCA1 or BRCA2 mutation
(Individuals of Ashkenazi ancestry)
Family History
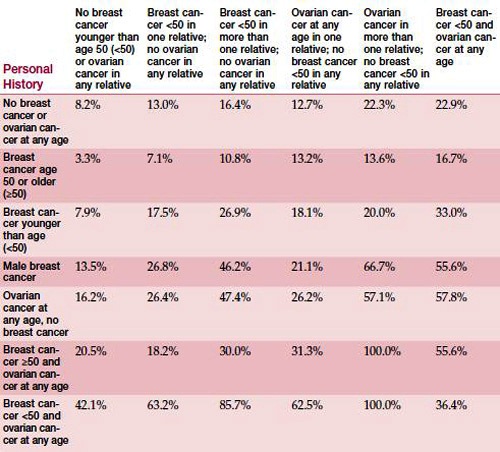
Adapted from BRCA1 and BRCA2 Prevalence Tables. Myriad Genetic Laboratories, Inc.
How much does testing cost?
For many women, the cost of genetic testing is a major concern. The price varies, depending on the situation. For a woman who’s the first person in her family to be tested, the test currently costs about $3,500. This is because BRCA1 and BRCA2 are very large genes and a mutation could be present along their entire lengths. If the woman tests positive for a specific genetic mutation, then another relative in her family can be tested for that specific mutation at a lesser cost, generally about $500. The testing is less costly for other family members because the geneticists know exactly where to look for the mutation.
For women of Ashkenazi Jewish descent, the initial test screens for the three most common genetic alterations in the Ashkenazi population, which account for 80 to 90 percent of hereditary cases of breast and ovarian cancer in Ashkenazi Jewish women. The cost for this initial test is approximately $600. If the result is negative, a woman may choose to have more extensive testing done.
Many insurance plans cover the cost of genetic testing or part of it. But some health plans don’t, and some women aren’t comfortable having their insurer know they’re being tested. While many women are concerned that genetic testing could lead to insurance discrimination, there are few known instances in which this has occurred. To learn what your options are, talk to your doctor or insurer.
Whether to undergo genetic testing is a personal decision. Many women proceed and feel relieved to know their risk status, even if they have a mutation, because this knowledge makes them feel empowered. They believe that the information they’ve gained will help them make the right decisions. Others worry about discrimination from health insurers or employers or about the cost of testing. For most women, the issue of genetic testing is emotionally charged, which is why counseling is critical.
Following are some factors to consider as to how you may react to either a positive or negative test result. If you decide to be tested, the appropriate test will be selected and ordered, and your blood will be drawn. The test is a simple blood test. It generally takes about three to four weeks to get the results. The counseling team will likely discuss your results with you in person.
≈≈≈≈≈≈≈
When genetic testing for BRCA1 and BRCA2 is done, you don’t always get a clear-cut answer. Sometimes, there’s an alteration in a gene but scientists don’t know if the alteration leads to a defective protein.
To understand this, it’s important to know that the molecular spelling of all of our genes differs slightly from one person to another. However, these minor changes don’t affect the function of the protein that’s produced from the gene — the protein still does what it’s supposed to.
In between these minor changes, which aren’t harmful, and over 1,500 genetic mutations that are known to be harmful, lies a gray area of genetic changes called variants of uncertain significance (VUS). About 5 to 20 percent of the time, genetic testing of BRCA1 and BRCA2 will produce a VUS result. This means that the laboratory cannot determine if the gene change is a harmless one or harmful one.
This is a complex area where genetic counseling can be very helpful, partly because the individual who was tested may be confused or frustrated with the results. People who receive VUS results should stay in contact with their genetics specialist. As further research is done, these variants may be reclassified as harmful or harmless.
≈≈≈≈≈≈≈
Positive test results
If genetic testing reveals a BRCA gene mutation, you might experience a range of responses to learning your test results:
Negative test results
For women in families with a BRCA1 or BRCA2 mutation who test negative for the mutation, there can be various reactions:
![]()

Jill, left, and Janet, right, following their cancer treatments. ©MFMER
One Family’s Story
Here is one story of a family with hereditary cancer that was revealed over a several-month period.
When Janet noticed a dimpling under her left breast while doing her monthly breast self-exam, she knew it was cancer, though she had no reason to suspect it. Janet was only 38 years old, a runner and in good health. She didn’t smoke, didn’t drink and wasn’t aware of any family history of breast cancer — because Janet’s mother was adopted, the health history on that side of the family was unknown.
Being a physician, Janet was only too aware that dimpling is a common sign of breast cancer. Then there was also the mild pain she had been experiencing while breast-feeding her youngest son, something she had easily dismissed to nursing — until now. What she didn’t know standing in front of a mirror that November day was how her discovery would profoundly change not only her life but also the lives of four other women.
Janet immediately saw her doctor. A mammogram and additional tests confirmed her fears. It was cancer. While awaiting the final test results, Janet sat in her husband’s lap and cried, agonizing over how her young sons would cope without her. “In my mind, I just went right to the worst-case scenario, that I had stage IV cancer.”
Fortunately, the cancer was only at stage II. Janet had her left breast removed, followed by a regimen of chemotherapy, radiation and the hormone medication tamoxifen.
Not surprisingly, Janet’s diagnosis came as a shock. She grew up in a tightknit family, and her parents and three sisters were devastated when Janet called with the news. Her younger sister, Jill, drove from Michigan to Minnesota to be with Janet and help take care of the boys during Janet’s first round of chemotherapy.
At Janet’s insistence, her sisters each made appointments to have mammograms. The news was good; the results came back fine. A sigh of relief, but unfortunately it would be short-lived.
It was less than six months after Janet’s diagnosis when her sister Jill experienced a perplexing event. A young child jumped into Jill’s arms, accidentally kicking her in the breast. The incident caused intense pain, and it bothered Jill that such a minor mishap should bring about such pain. Later, while examining her breasts at home, Jill felt a lump — the same one she first noticed before her recent mammogram. Her doctor had told her then that the lump was likely a cyst and not to worry about it. And, for a while, Jill didn’t, having been reassured by the “normal” finding on her mammogram that everything was OK. But Jill didn’t like that the lump hadn’t gone away and that it didn’t seem to move. To be safe, she made another appointment to see her doctor. Still feeling it was just a cyst, Jill’s doctor ordered a biopsy to comfort Jill’s concerns. The results weren’t comforting. It was cancer.
That summer, while Janet was beginning her radiation therapy, 31-year-old Jill underwent a mastectomy. For Jill, the diagnosis was more severe. The tumor was close to 8 centimeters, and she had several involved lymph nodes — a stage III cancer. Her treatment would be even more extensive than Janet’s.
Janet made plans to travel to Michigan to be with Jill during her initial round of chemotherapy. Though tired from her radiation treatments, Janet was determined to be there for Jill, just as Jill had been there for her. Shortly before she was getting ready to leave, Janet’s mother, Charlotte, called. The call didn’t turn out to be the routine, “Hi, how are you doing?” Charlotte had just been in for a mammogram. She had had previous mammograms, but this was the first since her two youngest daughters had been diagnosed with breast cancer. The results showed an “axillary abnormality.” She didn’t know what it meant. Janet did. Her mother had an enlarged lymph node under her arm, likely due to breast cancer. For Janet, the news was almost too much to bear. “I was talking to her on the phone and I said, ‘I cannot take another diagnosis of cancer right now.’ This was literally weeks after Jill’s diagnosis.” Within a few days, 71-year-old Charlotte underwent surgery to remove a small cancerous mass in her breast that had spread to lymph nodes under her arm.
For the two oldest sisters, Julann and Jean, it suddenly felt as if their breasts had turned into ticking time bombs. Julann made an appointment with a surgeon to discuss the possibility of preventive (prophylactic) mastectomy. Though genetic tests showed that the family didn’t carry the BRCA1 or BRCA2 breast cancer gene, it was apparent that some inherited genetic abnormality was at play, which current technology can’t yet identify. After a battle with her insurance company, 46-year-old Julann finally received approval for surgeons to remove and reconstruct both of her breasts. As Janet sat in the waiting room and received updates from the operating room nurse, she became worried. The general surgeon was still in the room. “He should be done by now,” Janet thought. “This isn’t good.” The surgeon would later tell Janet what she already suspected. During the surgery, the surgical team found cancer. Fortunately, it was still in its early stages.
For the fourth time in just over a year, cancer had raised its ugly head in this family. For these women, their once-normal routines became vague recollections. Janet recalls a single week in January. Her mother was in the hospital, Jill was just completing radiation treatments, Julann was starting chemotherapy, and Jean was going in for a breast biopsy because a mammogram had revealed an abnormal mass.
Finally, for Jean, there would come a break. Her biopsy results came back negative. But she decided there would be no more waiting, no more gambling with time. She decided to have prophylactic surgery.
As they did during those long and often grueling months, faith, family and a sense of humor continue to keep the women going today. “As hard as it was, sometimes you just had to step back and laugh,” says Jill. “If you didn’t laugh about it, you would cry all the time.”
The women remain cancer-free. They credit their good fortune to the fact that they were proactive. They performed self-exams, had regular mammograms and took preventive action.
![]()