I. ATOMIC THEORY—THOUGHTS ON THE STRUCTURE AND NATURE OF THE ATOM SPAN MANY CENTURIES
A. EARLY IDEAS
1. Circa 400–5 BCE Greek philosopher Democritus proposes the idea of matter being comprised of small, indivisible particles (atomos).
2. Late 18th Century. Lavoisier proposes the law of conservation of mass, i.e., that matter cannot be created or destroyed. Proust proposes the law of definite proportions (or the law of constant composition), i.e., that a given compound will always contain the same proportion of chemical elements by mass.
B. DALTON’S ATOMIC THEORY
1. In the early 19th century, using the previously somewhat unconnected ideas discussed earlier, Englishman John Dalton formulated his atomic theory.
i. Matter is composed of tiny particles called atoms.
ii. All atoms of a given element are identical.
iii. The atoms of a given element are different from those of any other given element.
iv. Atoms of different elements combine in small, whole number ratios to form compounds. A given compound always has the same relative numbers and types of atoms (law of definite proportions), but different compounds containing the same elements can be formed, by combining elements in differing small whole number ratios (the law of multiple proportions). For example, 12 g of carbon can combine with 16 g of oxygen to form carbon monoxide, CO, but 12 grams of carbon can also combine with 32 g of oxygen to form carbon dioxide, CO2. The ratio of oxygen masses that combine with 12 g of carbon is 16:32, i.e., 1:2, a simple, whole number ratio.
v. Atoms cannot be created or destroyed in a chemical reaction—they are simply rearranged to form new substances (law of conservation of mass).
C. EXPERIMENTAL EVIDENCE FOR THE ATOMIC MODEL
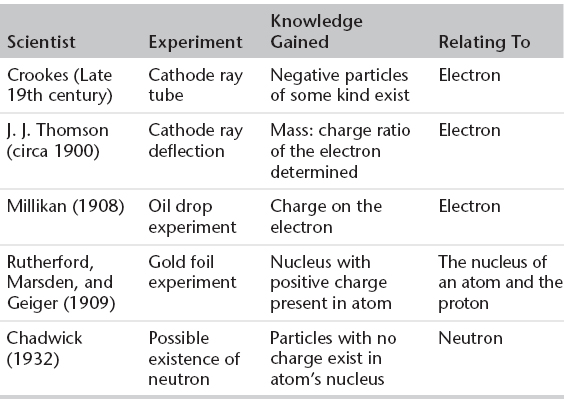
1. Several experiments were being carried out, and ideas formulated, in the late 19th and early 20th centuries that began to identify the subatomic particles that comprise the atom along with their position and nature.
Summary of Early Experiments Used to Formulate Atomic Theory

2. In the very early 1900s, the plum-pudding model (electrons dispersed in a “pudding” of positive charge) was the working model.
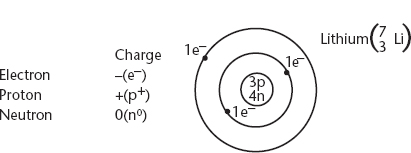
1. Niels Bohr took some of Rutherford’s ideas and proposed the idea that the atom was comprised of a dense nucleus containing protons and neutrons that was being orbited by the electrons in specific, allowed orbits. Sometimes called the solar system model, the atom was then thought of as:
i. A dense, central nucleus containing protons with a charge of +1, and a mass of 1 amu, and neutrons with a charge of 0 and a mass of 1 amu (amu = atomic mass unit).
ii. Electrons with a charge of −1 and a mass of approx. 1/2,000 amu that are widely dispersed in specific positions outside of the nucleus. See the following diagram.
2. Schrödinger, de Broglie, and Heisenberg introduced wave functions and quantum mechanics to account for the fact that electrons exhibit properties that are associated with waves as well as being particles.
II. SYMBOLS, ATOMIC NUMBERS, MASS NUMBERS, AND ISOTOPES
A. EACH ELEMENT HAS A ONE- OR TWO-LETTER SYMBOL ON THE PERIODIC TABLE, AND TWO NUMBERS ASSOCIATED WITH IT (E.G., CARBON CAN BE DEPICTED AS 6C12). THE SMALLER NUMBER IS CALLED THE ATOMIC NUMBER AND THE LARGER THE MASS NUMBER
1. Atomic number (Z) = the number of protons (positive particles) in the nucleus of one atom of that element. Because all atoms are neutral (carry no electrical charge), it also tells us the number of electrons (negative particles) in the atom.
2. Mass number (A) = number of protons + number of neutrons (neutral particles).
3. Isotopes. The number of protons and neutrons in atoms are always whole numbers, and as such one might expect the mass number to always be an integer, too. However, many elements have mass numbers recorded on the periodic table as noninteger values. Why?
i. Atoms of the same element are defined by the number of protons that they contain; for example, atoms of carbon always have 6 protons and atoms of oxygen always have 8, and so on.
ii. The number of neutrons present can vary and hence the mass numbers of the same element can vary, too.
iii. Atoms of the same element with different numbers of neutrons are called isotopes.
iv. The reported atomic mass is the average mass of all of the naturally occurring isotopes of an element, and so the mass number can be a noninteger value.
Example question:
Chromium has four naturally occurring isotopes—50, 52, 53, and 54—in abundances of 4.350%, 83.79%, 9.500%, and 2.360%, respectively. What is the average atomic mass of chromium?
(A) 48.06
(B) 50.06
(C) 52.06
(D) 54.06
(E) 55.06
Answer: (C)
The detailed calculation is (50*0.04350) + (52*0.8379) + (53*0.09500) + (54*0.02360) = 52.06.
This is a good example of a question that should remind you of two things:
1. No calculators are allowed on the test, so you will need to be able to do some simple, mental arithmetic and use estimation.
2. You should check all of your numerical answers to see if they make sense.
In the case of average atomic masses, the correct answer must be somewhere between the highest and the lowest isotopic mass, and must be closest to the most abundant isotope. Answers that do not fulfill these criteria do not make sense and you should think again.
Applying this simple, “Does it make sense?” test to all other numeric answers on the test will help you to identify any silly mistakes.
III. IONS
A. ATOMS BECOME IONS WHEN THEY CEASE TO BE NEUTRAL AND BECOME CHARGED BY EITHER LOSING OR GAINING ELECTRONS
1. Electrons are either removed from, or added to, the valence shell of that atom, i.e., the shell that is furthest from the nucleus.
2. Cations are positive and are formed by losing electrons, e.g., when an Na atom (11 protons, 11 electrons) loses an electron to become an Na+ ion (11 protons, 10 electrons).
3. Anions are negative and are formed by gaining electrons, e.g., when an S atom (16 protons, 16 electrons) gains 2 electrons to become an S2− ion (16 protons, 18 electrons).

The history behind the discovery of subatomic particles and the development of atomic theory is generally not examined heavily on the test. Because of this, you should focus your study and review on knowing the subatomic particles, their position and nature, and understanding isotopes and ions. Do not be concerned with a history lesson where a collection of dates and scientists’ names need to be learned. However, you should be familiar with the broad ideas behind the development of atomic theory.