I. SOLUBILITY OF SOLIDS AND LIQUIDS
A. TEMPERATURE AND PRESSURE CONSIDERATIONS
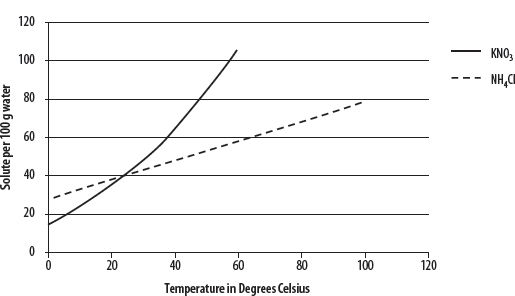
1. In general, solids and liquids become increasingly soluble with increased temperature of solvent. See the following diagram.

2. When no more solute will dissolve, the solution is said to be saturated.
3. Up to the point of saturation, i.e., when it is still possible for more solute to be dissolved, the solution is said to be unsaturated.
4. When conditions of temperature or pressure are artificially altered, it may be possible to dissolve more solute than one would otherwise expect in the solvent, i.e., to go beyond the point of saturation. When this happens, the solution is said to be supersaturated.
B. PARTICLE SIZE CONSIDERATIONS
1. Although particle size will influence the SPEED of a solid dissolving, it is not responsible for determining whether a solid will (or will not) dissolve. In that respect, particle size does not affect solubility.
C. AGITATION (STIRRING) CONSIDERATIONS
1. As in particle size considerations, agitation will influence the speed of dissolving but not the amount of solute that will dissolve. In that respect, particle size does not affect solubility.

There is a difference between how MUCH solute can dissolve and how FAST the solute dissolves.
II. SOLUBILITY OF GASES
A. TEMPERATURE CONSIDERATIONS
1. In general, gases become decreasingly soluble with increased temperature.
2. As temperature increases, the gas particles gain energy and can leave the solution more easily, meaning that less of the gas will be dissolved in the solvent and that the concentration of gas in solution will be lower.
B. PRESSURE CONSIDERATIONS
1. In general, gases become increasingly soluble with increased pressure.
2. As pressure increases, more gas particles strike the surface of the solvent and enter the solution, meaning that more gas is dissolved and that the concentration of the gas in solution will be higher.
3. Henry’s law states that at constant temperature and with a constant mass of gas, solubility is directly proportional to pressure.
i. ![]()
ii. If the solubility and pressure of a gas are known initially, and one of the variables is changed, then the new conditions can be calculated by using ![]()
(S1 and P1 are the original conditions and S2 and P2 are the new conditions. Units of solubility and pressure must be the same on each side of the equation.)

Think about a bottle of soda (a fizzy drink). When is the soda most “fizzy,” i.e., when does it have most carbon dioxide gas bubbles dissolved in it? It will be most fizzy when it is cold and under pressure. When it is warm and opened (no longer under pressure) the gas is least soluble in the drink and it escapes, making the drink “flat.” That’s a summary of Henry’s law!