
I. PRECIPITATION REACTIONS
A. PRINCIPLES AND SOLUBILITY RULES
1. Some ionic compounds are very soluble in water, while others are less so.
2. A precipitation reaction occurs when a double displacement (double replacement) reaction takes place between two aqueous solutions, and certain cation and anion combinations lead to the formation of an insoluble compound. The insoluble compound is called a precipitate (ppt.).
3. The solubility rules allow predictions about which combinations of ions will form precipitates.
Solubility Rules
| SOLUBLE | INSOLUBLE |
| Group 1 and ammonium | Hydroxides |
| Nitrates, hydrogen carbonates, chlorates, ethanoates, and perchlorates | (EXCEPT Group 1 and ammonium; hydroxides of Ca2+, Sr2+, and Ba2+ are slightly soluble) |
| Chlorides, bromides, and iodides (EXCEPT Pb2+, Ag+, and Hg2 2+) | Carbonates, phosphates, sulfites, chromates, and sulfides |
| Sulfates (EXCEPT Pb2+, Ag+, Hg2 2+, Sr2+, Ba2+, and Ca2+) | (EXCEPT Group 1 and ammonium; sulfides of Group 2 are soluble) |

You must commit these solubility rules to memory. They are a crucial piece of knowledge that you must carry with you into the test.
B. FULL EQUATIONS, IONIC EQUATIONS, AND NET IONIC EQUATIONS—Consider the reaction between an aqueous solution of silver nitrate and an aqueous solution of sodium iodide.
1. Full equation. A double displacement reaction takes place. Solubility rules predict that the silver iodide is insoluble and therefore will form a precipitate. The same rules predict that sodium nitrate will remain in aqueous solution because it is soluble.
AgNO3(aq) + NaI(aq) → AgI(s) + NaNO3(aq)
2. Ionic equation. An ionic equation shows all the aqueous ions fully ionized in solution, and any insoluble solids in a nonionized state.
Ag+(aq) + NO3−(aq) + Na+(aq) + I−(aq) → AgI(s) + Na+(aq) + NO3−(aq)
3. Net ionic equation. In the ionic equation, the sodium and nitrate ions are in the aqueous state on both sides of the equation and do not take part in the reaction because they remain unchanged. They are said to be “spectator ions” and they can be canceled out.
Ag+(aq) + NO3−(aq) + Na+(aq) + I−(aq) → AgI(s) + Na+(aq) + NO3−(aq)
This leaves an equation that shows only the ions that take part in the reaction. This is called the net ionic equation.
Ag+(aq) + I−(aq) → AgI(s)
C. IDENTIFICATION OF PRECIPITATES IN QUALITATIVE CHEMISTRY—The formation of colored precipitates is an important tool in aqueous qualitative chemistry. The following table summarizes a few of the more common reactions that are used in qualitative analysis.
Qualitative Tests for Some Anions and Cations
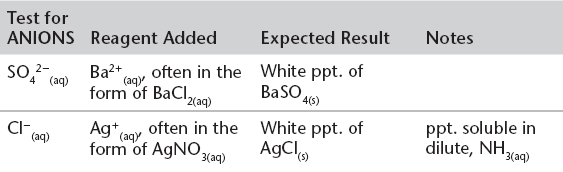
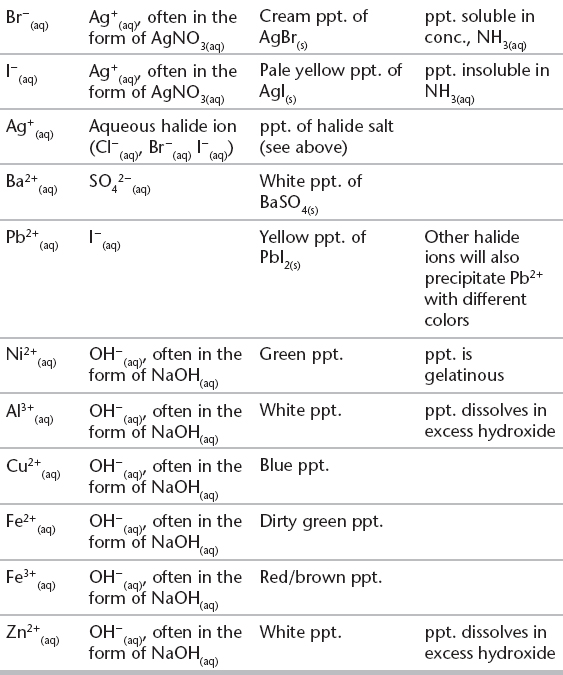

The recognition of some common colors can be a really useful tool in “unlocking” some questions on the test. Try to remember as many as possible.