
I. PERCENTAGE BY MASS COMPOSITION
A. CALCULATION OF PERCENTAGE BY MASS COMPOSITION
1. To determine the percentage by mass composition of an individual element within a compound, simply express the mass of each element as a percentage of the total mass of the compound.
2. See the following example.
i. A compound with the formula C2H4O2 has a total mass of (2)(12.011) + (4)(1.0079) + (2)(16.00) = 60.05.
ii. Percentages of each element, expressed as a function of the total mass, are shown as follows.

II. EMPIRICAL FORMULA
A. DEFINITION
1. The empirical formula of a compound is the simplest whole-number (integer) ratio of the atoms of each element in that compound.
1. Empirical formulae can be calculated from percentage by mass data.
2. See the following for the method for calculating empirical formula from percentage by mass data.
i. Take the percentage of each element and assume a sample of 100 g. (This assumption converts percentages to masses.)
ii. Convert the masses of each element to the moles of each element by dividing each element’s mass by the corresponding atomic mass taken from the periodic table.
iii. Find the ratio of the moles calculated in (ii) by dividing each of the moles by the smallest number of moles.
iv. The results from (iii) will be in a convenient ratio and gives the empirical formula.

It may be that the ratio includes a decimal (fraction) such as 0.500, 0.333, 0.250, and so on. If so, then because empirical formulae are simplest, WHOLE-number ratios, you must multiply all of the numbers in the ratio by 2, 3, or 4 as appropriate in order to remove the decimal.
3. Following is an example of an empirical formula calculation. Calculate the empirical formula of a compound containing 40.1% carbon, 6.70% hydrogen, and 53.3% oxygen by mass.
Calculation of an Empirical Formula
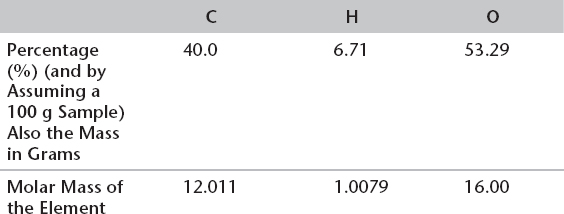

When performing empirical formula calculations, avoid rounding up or down too much in the middle of the calculation, and be lenient with significant figures.
III. MOLECULAR FORMULA
A. GENERAL PRINCIPLE
1. Unlike the empirical formula (which shows the simplest whole-number ratio), the molecular formula of a compound shows the exact whole-number ratio of the different elements in a compound.
2. Like the empirical formula, the numbers of each element are recorded using a subscript to the right of the element’s symbol. When only 1 atom is present, the subscript 1 is assumed (understood), and not written.
B. THE FOLLOWING DESCRIBES THE RELATIONSHIP BETWEEN THE MOLECULAR FORMULA AND THE EMPIRICAL FORMULA
1. The molecular formula will be some simple, integer multiple of the empirical formula. For example, a compound with an empirical formula of CH2O will have a molecular formula of either CH2O, or C2H4O2, or C3H6O3 and so on, where the empirical formula is multiplied by either, 1, or 2, or 3, and so on.
2. To establish the correct multiplier, and therefore find the molecular formula, it is necessary to know the molecular mass of the compound.
i. In the preceding example, given a molecular mass of 60, divide the molecular mass of the compound by the mass of the empirical formula. In this case, the mass of the empirical formula, CH2O is as follows.
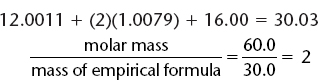
ii. The answer to i. is the multiplier, so (2)(CH2O) = C2H4O2 is the molecular formula.