
I. SPECIFIC HEAT CAPACITY
A. DEFINITION
1. The specific heat of a substance can be defined as the energy required to raise 1 g of that substance by 1°C (1 K).
B. CALCULATIONS
1. Calculations involving specific heat use the equation q = m c ΔT, where q = energy, m = mass, c = specific heat capacity, and ΔT = temperature change.
2. Following is an example calculation.
i. If 30.0 grams of silver absorbs 375 J of energy, and the initial temperature of the silver is 23.0°C, calculate the final temperature of the silver. The specific heat capacity of silver = 0.235 J/g°C.
q = m c ΔT
375 J = (30.0 g) (0.235 J/g°C) (ΔT)
ΔT = 53.2°C
Because the energy was absorbed, the temperature of the silver went up, so the final temperature = 23.0 + 53.2 = 76.2°C.

Because ΔT represents a change in temperature, and a change of 1 K is the same as a change of 1°C, then the units of temperature are interchangeable in these problems.
A. COFFEE-CUP CALORIMETRY
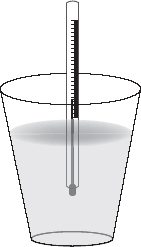
1. Styrofoam cups are commonly used as insulated containers in school laboratories for calculations involving specific heat capacities. See the following diagram.
B. EXAMPLE CALCULATION
1. If 5.00 g of urea are added to 90.00 g of water in a coffee-cup calorimeter, the temperature of the contents of the cup falls by 3.100 °C. If the specific heat capacity of the solution is 4.184 J/g°C, calculate the energy change in the coffee cup.
q = m c ΔT
q = (95.00 g) (4.184 J/g°C) (3.100°C)
q = 1232 J

Sometimes questions may ask for the energy change, q, to be expressed in units of J mol−1 or kJ mol−1. As such, be aware for the possible need for some simple unit conversions. Also, the specific heat capacity of water (4.184 J/g°C) is a very common value in these types of calculation; it helps if you can recognize it as such, and know that water has an unusually high specific heat capacity caused by hydrogen bonding (see Chapter 9).