
I. ORGANIC CHEMISTRY
A. WHAT IS IT?
1. Organic chemistry involves the study of compounds of carbon. There are many compounds containing carbon and hydrogen only and millions of other organic compounds containing carbon, hydrogen, and other elements such as nitrogen, oxygen, sulfur, and the halogens.
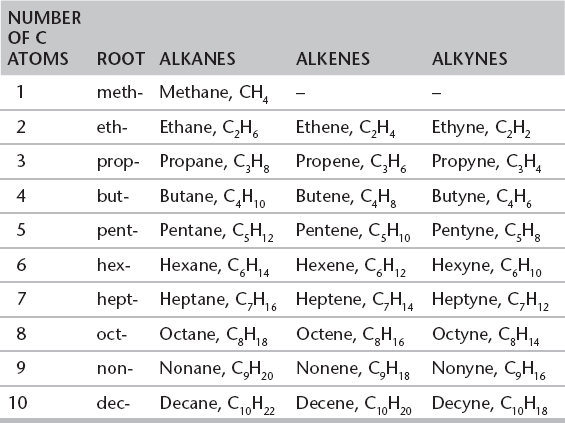
II. HYDROCARBONS AND NOMENCLATURE
A. HYDROCARBONS
1. A hydrocarbon is a compound containing hydrogen and carbon only. Following are three common types.
i. Alkanes with the general formula CnH2n+2 that contain all single bonds (organic compounds that contain all single bonds are said to be saturated)
ii. Alkenes with the general formula CnH2n that contain carbon–carbon double bonds (organic compounds that contain double or triple bonds are said to be unsaturated)
iii. Alkynes with the general formula CnH2n−2 that contain carbon–carbon triple bonds (organic compounds that contain double or triple bonds are said to be unsaturated)
(In all of the preceding general formulae, n = the number of carbon atoms in the compound.)

Single bonds between atoms are called sigma (σ) bonds. A double bond is comprised of one sigma and one pi (π) bond, and a triple bond is comprised of one sigma and two pi bonds.
B. NOMENCLATURE—NAMING HYDROCARBONS
1. The root name of any organic compound is based upon the number of carbon atoms in the longest, continuous carbon chain.
2. The suffix (ending) is based upon the type of hydrocarbon. Alkanes have an “-ane” ending, alkenes “-ene,” and alkynes “-yne.”
Nomenclature of Hydrocarbons
A. TYPES
1. When organic compounds contain atoms other than just carbon and hydrogen, other functional groups exist. A functional group is a specific group of atoms within an organic molecule that is responsible for the chemical properties of that molecule.
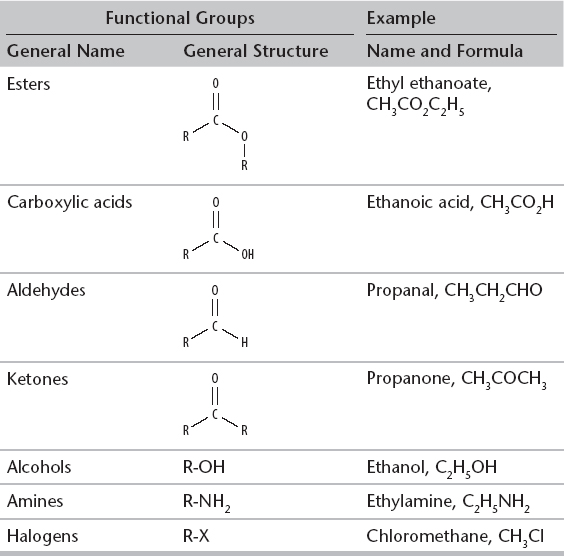
R = a carbon chain and X = halogen
A. ISOMERISM
1. Isomers are compounds with the same molecular formula but different molecular structures or arrangements in space.
2. This can lead to a number of different structures with the same molecular formula.
3. The structures can have different functional groups, the same functional groups but with different bonds present, or different physical arrangements in space.
4. A compound that contains a carbon atom with four different groups attached to it can exhibit optical isomerism. These compounds have nonsuperimposable mirror images.
B. EXAMPLES OF PAIRS OF ISOMERS
1. Pentane and 2-methylbutane—both C5H12:
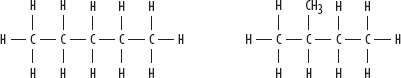
2. Butan-1-ol and butan-2-ol—both C4H9OH:

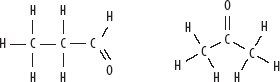
3. Propanal and propanone—both C3H6O:
4. Optical isomers with nonsuperimposable mirror images—e.g., 1-choroethanol where a central carbon atom has
(a) hydrogen, (b) methyl (CH3), (c) hydroxyl (OH), and (d) chlorine attached—both C2H5OCl.
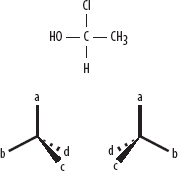

If a question asks if a pair of compounds are isomers of one another, simply add up the number of carbon, hydrogen, oxygen, etc., atoms in each compound, and if the numbers of each type of atom are the same and those atoms are arranged in different ways, then they are a pair of isomers. These molecules are mirror images of one another, and rotate plane-polarized light in different directions.