The biopsychology of motivation
Using evidence from neurology and endocrinology to understand motivated behavior
This chapter describes the relationship between human neuroanatomy and selected motivations that underlie learning and performance. Although specific biopsychological correlates of motivation are debated, strong multi-disciplinary evidence reveals that incremental changes within the human nervous and endocrine systems are correlated with organized behavior. First, a myriad of interpretive issues regarding physiological data are presented, along with practical applications for the use of biopsychological findings. Next, key findings relating to variables commonly believed to subsume performance motivation are discussed, including biological correlates of power, affiliation, achievement, pleasure, and pain. Finally, the heritable and evolutionary nature of motivated behavior is reviewed.
Keywords
Neurology; biopsychology; endocrinology; brain research
Did you look in the mirror today? What did you see? Perhaps your attention was drawn to a blemish on your face or to your disheveled hair upon arising. Instead, maybe you embraced a more holistic view of your physical being and were beaming with confidence as you assessed the results of a recently implemented dieting or exercise regime. Most likely, the subjective appraisal of your body did not include an evaluation of the 180 billion cells that comprise a complex network of neurotransmitters, synapses, and hormones that collectively encompass your neuroanatomy (Kolb & Whishaw, 2009). However, the precise composition of your brain, with an emphasis on your nervous and endocrine systems, is arguably one of the most reliable predictors of ensuing behavior, more consistent than many self-reported descriptions of motivation (Falk, Berkman, Mann, Harrison, & Lieberman, 2010). Perceptions and self-reflections about how the mind and the body interact are the essence of over 2,000 years of ardent philosophical and scholarly ruminations concerning the etiology of human behavior.
Historically, classical thinkers, such as Socrates, his student Plato, and Plato’s student Aristotle, contended that the conceptual framework of human behavior was guided by three separate and distinct forces: the body, the mind, and the soul. Socrates held the unpopular belief that human “will” mediated and controlled bodily actions. Lacking any hard evidence to support his beliefs, Socrates was eventually shunned by the majority of the secular ancient Greek populace and suffered the fate of death by poison hemlock for his seemingly radical views. Protégé Plato had similar contentions as his mentor, but he differed in the exact descriptions and terminology that he used to describe motivated behavior. Unlike Socrates, who took a conciliatory approach to the influences of reason and spirit as mediating the carnal urges of the body, Plato believed that a divisive and eternal conflict vexed the human psyche (Singpurwalla, 2010). Aristotle advanced the views of Plato, placing a greater emphasis on logical interpretations while still believing that the mind was guided by a combination of competing urges. Aristotle’s views on motivated action led to the revelation that behavior was attributable to seven causes: chance, nature, compulsion, habit, reason, passion, and desire (Aristotle, 2004). Collectively, the deterministic views of the ancient scholars all sought to answer the same pressing question: Which metaphysical aspects of human existence accounted for motivated behavior?
The tripartite view of the mind perpetuated until the seventeenth century when Descartes, the noble laureate and inventor of Euclidian mathematics, declared, “Cogito ergo sum” (“I think, therefore I am”). The metaphysical argument advanced by Descartes led to the enduring concept of dualism. This interpretation of human existence hypothesized that the body and the mind (centered in the brain) were physically distinct, qualitatively different, and yet, somehow, worked together (Kolb & Whishaw, 2009). Descartes never effectively explained how the body and mind interacted and mistakenly thought that the pineal gland, which regulates the hormone melatonin, was the moderator of thought and action. Despite the biological imprecision advanced by Descartes, his dualistic philosophy launched the study of biopsychology and influenced the origins and explanations of motivated behavior for well over 300 years. Today, the discipline of biopsychology extends the views of Descartes and focuses on substantiating how the brain and the neurological system intertwine, determining which areas of the brain activate motivations, and clarifying the meaning of the behavioral expressions triggered by physiological events.
Principle #13—Neurological/endocrinological evidence informs or refutes behavioral evidence
First, let’s consider how biobehavioral evidence helps the motivational detective (MD) understand motivated behavior. Can evidence garnered through biopsychological sources have any utility for a teacher or leader? For example, does it really matter what part of your brain is activated when you are anxious about an upcoming interview or test? Although your initial reaction may be a resounding “NO,” from an applied perspective, biopsychological evidence helps either support or refute the contentions advanced by observation or individual self-report alone. However, the interpretation of biopsychological evidence is complex due to the hierarchical and interactive nature of the neurological system and because behaviors rarely align exactly with specific anatomical changes.
Biopsychological evidence can be interpreted in at least four ways. Figure 3.1 depicts the nexus of four possible outcomes when interpreting the relationship between behavioral and biological evidence. In cases when homogeneous behaviors result in predictable physiological reactions, the confluence of evidence suggests that the observed behaviors are a consistent and accurate reflection of individual motives. When heterogeneous behaviors result in predictable physiological reactions, we might conclude that individual differences or contextual variables (e.g., social factors) have a prevailing influence on determining underlying motivations. Conversely, if behavioral heterogeneity is observed in association with unpredictable or novel physiological changes, the likely interpretation would be that the exhibited behaviors are related to a diverse set of motivations. Finally, inconsistent behaviors that show little correlation to physiological changes would represent no specific relations between behavior and motivation. The ongoing scrutiny of behavioral triggers and associated biological patterns becomes instrumental in accurately interpreting what the diverse behaviors encountered in the classroom or workplace actually represent. Thus, attention to physiological markers, such as facial expressions, speech tones, or breathing patterns, can prove valuable, falsifying otherwise sound behaviorally determined explanations of motivated behavior.
A practical example demonstrating the functional utility of physiological evidence is the largely involuntary and innate physiological response to conscious fear. Using brain imaging technology, such as functional magnetic resonance imaging (fMRI) or electroencephalography, fear manifests neurologically by accelerating activation patterns in different areas of the brain, including the amygdala, insula, thalamus, and the occipital cortex, concurrent with the release of the neurotransmitter serotonin (Klucken et al., 2013). Typical human behavioral reactions to fear include freezing, pupillary dilation, facial constriction, decreased salivation, and not surprisingly, as any frightened individual will attest, increased urination and a rapid heart rate (Davis, 1992).
I regularly encounter a potential fear-inducing stimulus on my street: His name is Gunner, a handsome, yet unpredictable white German Shepherd. Since I have been bitten by dogs at least three times, I approach Gunner with cautious apprehension. I never know if my tail-wagging buddy will afford me affection or growl in disdain when he is having a bad day. For argument’s sake, assume that I never leave my house unencumbered, always hooked up to my portable fMRI machine that monitors my brain functioning and fear responses. Immediately upon seeing Gunner my heart rate increases, my pupils dilate, and serotonin production increases (yet my pants remain dry). The fMRI flashes to show blood rushing to the fear receptor in my amygdala. Likely, one would conclude that Gunner is the cause of my fear-induced motivation, as both my physiological symptoms and my cautious and self-protective behavior are aligned. In order to further test my hypothesis of Gunner-induced fear, I lend my fMRI to 20 other monitored friends and ask them to approach Gunner. To my surprise, none of my friends shows brain-based fear localization or increased serotonin production. However, when approaching Gunner, some of them exhibit changes in facial expression and pupil dilation. Interpreting these results, we would likely conclude that the physiological reactions of my friends to Gunner are, indeed, different from my own, not involving the motive of fear. Curious about the observed results, I would feel compelled to ask my friends why they were not afraid of my nemesis, Gunner. As you might expect, many of them have dogs of their own, and the observed physical reactions were biological markers of affection. In this example, we can conclude that the nexus of neurological and behavioral evidence was significantly more helpful in understanding the nature of motivations than behavioral evidence alone.
Principle #14—Neurological/endocrinological inferences are multi-dimensional
Despite the logic advanced in the prior paragraph, neurological evidence should be interpreted cautiously for at least three reasons (Cacioppo & Berntson, 2009). First, as aspiring diagnosticians of motivated behavior, whenever possible, our aim is to decipher the causes of observed behavior. Regrettably, humans have a strong tendency to seek out remedies that require minimal investment of cognitive effort (Stanovich, 2009). In other words, given a choice, we prefer simple and quick explanations. The proclivity for simple solutions creates vulnerability to accept potentially spurious interpretations of data. This happens when causality is wrongly inferred to a particular variable based upon a correlational relationship, masking the true causal factor underlying the behavior of interest. For example, a confluence of neurological evidence indicates that males have elevated levels of testosterone when winning sporting events, playing chess games, and having success during games of chance, compared with when they experience defeat (Mazur & Booth, 1998). Women also show testosterone increases when participating in identical events, albeit to a far lesser extent than men. Analyzing these findings might lead to the widely accepted inference that biological sex causes testosterone production. However, this conclusion would, indeed, be spurious. In reality, contextual differences, such as the type of task performed and a number of social variables, including group dynamics, exerts a greater influence on testosterone production in both men and women, than sex alone (Hines, 2011).
Second, there may be a tendency to underestimate the immense complexity of neurological networks and associated hierarchies in the nervous system and to falsely attribute behavioral observations to a unitary neurological source. Interpretive errors may develop because unlike behavioral evidence, which is usually interpreted at the individual or group level, physiological observations can be interpreted at six different levels of specificity (Berntson & Cacioppo, 2008; Cacioppo & Berntson, 2009). Table 3.1 illustrates the perfect storm of potentially skewed interpretations of multi-level hierarchical neurological evidence. Each level can have an independent influence on behavior or multiplicative levels can have singular influences. Thus, looking at level alone is insufficient to evaluate the relationship between biopsychological evidence and behavior.
Table 3.1
Neuropsychology studies at different levels of neural activation
| Level of activation | Type of evidence | Source |
| Genetic | Genetic code of neurons | McConnell et al. (2013) |
| Synaptic | Visual attention | Lakatos, Karmos, Mehta, Ulbert, and Schroeder (2008) |
| Autonomic | Sensory perceptions, smiling behavior | Schilbach, Eickhoff, Mojzisch, and Vogeley (2008) |
| Skeletal | Reflexive actions, pain withdrawal | Rainville (2013) |
| Cognitive | Language syntax | Petersson, Folia, and Hagoort (2012) |
| Systems | Information processing | Rypma et al. (2006) |
Third, compounding accurate analysis of evidence is the potential interactivity of behavioral stimuli and neurological responses. Since the neurological system is structured in a complex hierarchy, at any given time, behavioral stimuli can activate one or more dimensions of the neurological hierarchy. McNaughton and Corr (2009) used the example of nine possible emotional responses to perceived threat, including increased respiration (autonomic), freezing (skeletal), and muscle energy in the form of adrenaline and glucose production (synaptic). The neuronal reactivity can be activated either independently or dependently of other neuronal pathways or more likely in a combined fashion, suggesting interactivity of responses. For example, we know from an environmental perspective that heat induces perspiration in humans; however, the degree of perspiration is moderated by humidity. Given a particular temperature, an individual may not perspire to the same extent, unless the humidity is above a certain threshold. Although both heat and humidity influence perspiration separately, interactive analysis provides much more important information to understand how heat and humidity together influence perspiration. Additional interpretive concerns arise when determining the sequences of neurological responses to activating stimuli. Any one neuronal reaction could be prompted at a particular time, one triggering the next; alternatively, the neurological responses can all fire simultaneously. McNaughton and Corr (2009) cautioned against assuming that “all stimuli activate a single neural representation of threat and this, in turn, activates the separate response systems” (p. 714).
Additionally, we must expect that neurological responses to motivation-provoking stimuli will generalize inconsistently across contexts. Although I periodically fear Gunner, I can assure you that I often show no fear response to other canines, but I always fear a charging rhinoceroses. Finally, due to the high incidence of correlational studies in neurological research, combined with our quest to determine causality of behavior, we should be consciously concerned about ambiguous temporal precedence (Shadish, Cook, & Campbell, 2002), a fancy term that illustrates the classic chicken versus egg dilemma. It may be unknown whether neurological reactions precede motivation, or vice versa, if the motivation precedes the reaction. Thus, I cannot tell if my heart beats faster because I fear my canine culprit Gunner, or if the faster beat of my traumatized ticker is responsible for my fear response (see Chapter 9 (p. 237) for a detailed discussion of motivation and emotion).
In total, these interpretive cautions suggest that biopsychological analysis of motivation can be an effective source of data for the MD. However, analysis of behavior should be undertaken not only in a generalized fashion (i.e., do animals evoke fear in humans?) but also at the individual level of specificity (i.e., does heart rate change based upon the size of the animal?) to accurately infer meaning from acquired data. Causal conclusions without this degree of scrutiny may, indeed, be unwarranted, resulting in the cardinal error of believing that factors are related when, indeed, they are not. As McNaughton and Corr declared, “the definition of a psychological construct should map to a specific aspect of a coherent neural and functional system” (p. 711). Cognizant of the interpretive liabilities when analyzing neurological data, we now turn to specific inferences from neurological studies that help understand motivation in applied settings, such as the classroom and the workplace, and many other aspects of our daily lives.
However, before moving forward, read the incredible story of a courageous and determined young lady, Alexis Paige Dixon, who, as a result of massive and unpredictable biological change, has transformed herself into a medical miracle and model of adolescent motivation.
Principle #15—The brain is a perceptual filter influencing subjective reality
I would like to encourage you to try a simple experiment. You do not need any fancy materials or equipment, just paper and pencil and a family member or friend. Turn on the television, or watch any YouTube video for 5 min. Pretend that you and your partner are crime scene investigators, and individually write down the most important details you noticed about what you viewed. Upon conclusion, compare your notes with your investigative partner. Likely, there were some things on your list that were not on your partner’s list, and vice versa. Perceptual interpretations are at the root of your discrepancies. Where you focused attention, the details you deemed important, and what words were used to describe observations can all be attributed to subjective reality imposed upon you by your brain.
What occurred in the experiment described above is the phenomenon of individualized representations of external perceptions. Philosophically, subjective reality implies that no two individuals have the identical perceptual lens. This notion is termed qualia, and the proponents of this notion of reality contend that virtually all visual, auditory, and essentially every other sensory input is imbued by the properties of conscious experience, creating individualized perceptions of external reality (Dennett, 1988). This esoteric approach to examining physical evidence accounts for how we explain the world around us. Physiological evidence supports the notion that reality is, indeed, subjective. If you have ever used a dog whistle you will know what I mean and quickly understand how the same stimulus can be interpreted differently based upon perceptual capability. The average human is able to detect sound between the approximate ranges of 16 hertz (Hz) (low pitch sounds) and 16,400 Hz (high pitch sounds). A canine’s hearing range is more expansive, approximated between 60 and 60,000 Hz. Dog whistles are crafted to produce pitches between 20,000 and 48,000 Hz, which exceeds the ability of human detection, so for us, the whistle is silent. Lack of auditory perception, of course, does not indicate an absence of sound, but it does mean that perceptual abilities across species are inconsistent due to brain differences.
Consequently, we acknowledge that perceptual realities will vary between individuals because “no two brains are identical” (Kolb & Whishaw, 2009, p. 149): ample neurological evidence supports this hypothesis. Differences in sensory acuity transcend hearing and influence the brain’s filtering process whenever sensory input is detected. Sensory stimuli are processed through a complicated regimen of neuronal gate-keeping, centered in the thalamus region of the brain. First, stimuli are evaluated, and then specific neurons are fired, modulated, and transferred to other brain areas for higher-order processing, if necessary. The degree of attention devoted to incoming stimuli, whether or not we acknowledge information to be worthy of our attention beyond initial perception, and even how we process information cognitively are all a function of perceptual filtering done through the thalamus area of the brain (Lee & Sherman, 2008).
More importantly, for our purposes, the brain’s perceptual filter has many applied implications. For decades, Elizabeth Loftus has examined the radical and real-life consequences associated with how the brain selectively filters, recalls, and interprets information. The brain can be tricked into believing propositions that have never occurred, such as during “suggestibility” studies, where false information is provided to participants in the hope that the messages will be so persuasive that individuals will believe the information to be true. Evidence reveals that the type and timing of investigative questions about an auto accident can affect the accuracy of witness’s recall, leading to memory alternations and potential wrongful convictions of perpetrators (Loftus, 1975). Other studies have induced humans to vehemently attest to remembering events that never happened, such as being lost in a mall (Loftus, 1997) and even recalling false, yet more traumatic events, such as being abused as a child (Laney & Loftus, 2013).
Suggestibility may also prompt positive motivations. Laney, Morris, Bernstein, Wakefield, and Loftus (2010) falsely led some adults to believing that they loved to eat asparagus when they were children. Subsequently, the same individuals completed surveys indicating that they would be more likely to eat asparagus (a healthy motivation) and pay more to purchase asparagus, compared with a control group that was not exposed to the false beliefs. Findings of this nature reveal the real-life consequences associated with how the brain processes and filters information. Findings also show how these memorial representations might possibly influence future behaviors based upon differences in how individuals process and respond to perceptual information. The message here is to exercise extreme caution when evaluating individual interpretations of seemingly straightforward events. What appears true for one may, indeed, be flagrantly false for another.
Principle #16—Neurological system organization facilitates or inhibits action
Evaluating the structure and organization of our neurological system is a study in contrasts. You may recall from high school biology that the nervous system includes the central nervous system (CNS), which comprises the brain and the spinal cord, and the peripheral nervous system (PNS), which encompasses a neural network throughout the body and organs that communicate with the brain. The PNS is further divided into the somatic nerve system, which is responsible for sensory reception in the nerves, organs, and skeleton, and the autonomic nerve system (ANS), which regulates involuntary bodily functions, such as heart rate and breathing. The ANS is further divided into the “antagonistic” (Beck, 2004, p. 44), yet symbiotic, parasympathetic nervous system and sympathetic nervous system (SNS), which are most relevant to understanding the range of behaviors and corresponding emotions that influence motivation.
The divisions of the ANS are largely responsible for the regulation of bodily energy. The SNS deploys energy in response to threatening stimuli, such as when you are anxious about taking a test or running away from the charging rhinoceros I described earlier. The perception of stress induces the body to release of a series of nonmonolithic, but correlated, hormonal and neural metabolites, such as testosterone, epinephrine, and cortisol. The hormonal changes during stress dilate pupils, trigger decrements in saliva production, elevate blood pressure, and accelerate heart rate. Concurrently, energy is redirected from the digestive and urinary systems in response to the “fight or flight” mechanisms needed to resolve whatever the brain perceives as stressful.
Assuming that you successfully outsmart or outrun the charging rhino, the parasympathetic system returns your body to a state of homeostasis through a gradual process of relaxation and hormonal stability evidenced by pupil constriction, increased production of saliva, and a slowdown in the circulatory and respiratory systems. The digestive and urinary systems would return to prestress states as the body offsets the physiological actions activated by the SNS. The primary neurotransmitter associated with the regulating activities of the parasympathetic system is acetylcholine. However, the neuropeptides progesterone and oxytocin (OT), which are related to some forms of social behavior, also accompany parasympathetic regulation (Gamer & Büchel, 2012). The competing nervous systems clearly show recognizable symptoms of motivated behavior that ebb and flow based upon how our brains perceive and evaluate environmental stimuli.
Lessons in neuroanatomy may seem unimportant to the MD, but surprisingly, the divisions of our neurological system in many ways parallel how we navigate the world around us. Although it is unlikely you will actually have a charging rhino in your classroom or boardroom, it is probable you will be exposed to a series of stressful events and disturbing people, metaphorically acting like charging rhinos or hissing snakes. A primary human motive is avoiding or minimizing stimuli perceived as aversive and stressful and to gravitate toward those circumstances or people deemed desirable. This dichotomous framework of rival motivations is described using a variety of polarized terminology, including “approach versus avoidance” (Berntson & Cacioppo, 2008, p. 193), “initiation versus prevention” (Kolb & Whishaw, 2009, p. 146), or “arousal versus inhibition” (Schultheiss & Burnstein, 2010). Collectively, these labels represent humans’ perpetual quest to take courses of action that provide pleasure and reward and avoid circumstances that interfere with our personal definitions of satisfaction. Which labels you use is secondary to the realization that behaviors routinely exhibited in the classroom and workplace surprisingly mirror hormonal and neurological triggers in the sympathetic or parasympathetic nervous systems. We next examine biological correlates of common motives related to learning and performance, including the needs for power, affiliation, achievement, and the dispositions of seeking pleasure and attaining overall well-being.
Principle #17—Power and social dominance displays mimic sympathetic nervous system activation
You don’t know the power of the Dark Side, I must obey my master.
Darth Vader, Star Wars
Universally, displays of power motivation abound. Teachers insist students complete homework, bosses demand increased productivity from their workers, and coaches dictate who will be in the starting lineup. As a teenager, I asked my mother (Mrs. Darth Vader), “Why do I have to go to college?” She firmly removed any doubt in my mind by calmly saying, “Because I say so, that should be good enough for you!” The display of power is a prominent theory of motivation that helps explain why some individuals defer to autocratic and controlling leadership and teaching styles as a means to influence performance (McClelland, 1975; Winter, 1973). Power motivation occurs when individuals assume that they have the ability to make decisions, control, or take actions to determine the behaviors of others (Magee & Langner, 2008). Reeve (2009) identified three criteria representative of a controlling motivational style: prioritizing your own perspective over the desires of others, deliberately attempting to convince others that your way is the best way, and the persistent application of pressure to induce behavioral change in subordinates. Individuals with a high power motivation are more aggressive and assertive and less willing to negotiate disagreements (Winter, 2000). Most importantly, self-proclaimed high-powered individuals, when exposed to experimental manipulations where power motivation can be readily displayed and measured, exhibit biological response patterns similar to those of people facing abject fear or a threat of harm.
The biological markers of the SNS, primed during displays of power and dominance, include increased production of the hormones epinephrine, norepinephrine, testosterone, and cortisol (Schultheiss & Brunstein, 2010). “Dominance” refers to the motivation of an individual to nonaggressively achieve or maintain a high social status (Mazur & Booth, 1998). The omnipresent effect of testosterone is usually associated with power and dominance, with diverse studies revealing that testosterone increases occur in both men and women when playing sporting events (Mazur & Booth, 1998; Schultheiss & Brunstein, 2010), during video game contests (Carré, Campbell, Lozoya, Goetz, & Welker, 2013), when chopping down trees (Trumble et al., 2013), and even vicariously among spectators watching sporting events (Bernhardt, Dabbs, Fielden, & Lutter, 1998). Testosterone increases are implicated in these types of studies because the competitive nature of the tasks provides an opportunity for individuals to display socially acceptable dominance as ancillary to completion of the task. Like the peacock spreading his plumage or the warbling canary, competition serves as the stage for humans to “strut their stuff” and show off their abilities. However, there is one caveat: Baseline testosterone routinely decreases when men lose competitions, but the same outcome is only sporadically observed in women. Although researchers provide many different explanations for this gender discrepancy, the most likely reason for differences may be the general passivity associated with female gender roles across cultures (Carré et al., 2013; Schultheiss et al., 2005).
Regardless of gender, increased levels of testosterone have repercussions for organized and motivated behavior. Foremost, testosterone lowers the threshold for aggressive behaviors (Schultheiss et al., 2005) and is related to greater social control, persistence, and combativeness (Dabbs, Alford, & Fielden, 1998). Besides the very obvious ramifications for more aggressive behavior while participating in sporting events, students or workers involved in competitions may display overzealous status seeking, or exhibit manic behaviors as part of their competitive spirit. Kohn (1999) outlined the detrimental consequences of classroom contests, including increased learner anxiety, a diminished sense of empowerment, and less individual accountability. Similarly, workplace competitions instill a weakened team focus in employees, creating animosity and alienation between those succeeding and those individuals that fail. Fortunately, increased testosterone is not all bad, as individuals showing elevated levels who achieve successful outcomes are prime candidates for focused engagement in future tasks and are more likely to achieve learning gains (Stanton & Schultheiss, 2009).
Cortisol is also related to the perception of stress, but with a special emphasis on perceived stress due to social factors. For example, some individuals under observation while performing mental arithmetic and giving speeches are more inclined to exhibit anxiety, freezing, or wanting to completely withdraw from a task compared to performing in isolation (Roelofs et al., 2009). In response to social stress, the adrenal gland releases the hormone cortisol; however, unlike testosterone, high basal cortisol levels are linked to anxiety and social avoidance. Conversely, low basal cortisol levels are linked to decreased stress and behaviors related to embracing social situations (Mehta & Josephs, 2010). Individuals with high cortisol levels have been found to be more inclined to exhibit dominance behaviors, such as repeatedly trying to beat the same opponent on a task. As for power motivation and cortisol, few studies exist, but Wirth, Welsh, and Schultheiss (2006) observed increases in cortisol after “high-power motivated” individuals lost a contest, but the same individuals did not have increased cortisol after winning. These results suggest that people high in power motivation will likely be stressed when they lose a contest or when they cannot complete a task, more so than individuals with other dominant motivations.
Although these laboratory studies provide useful insight into the relationship between hormonal releases and power motivation, these studies have been exclusively conducted in synthetic settings and leave unexplored the relation between different motivation styles and cortisol production. Experimental manipulation of motivation style while measuring hormonal production allows the researcher to determine what specific teaching and leadership approaches are perceived as stressful and which instructional strategies facilitate performance and engagement. In one of the few studies of its kind, Reeve and Tseng (2011) had educational psychology students solve puzzles while being exposed to either a controlling style of teaching, where a voice recorded instructor barked out solution strategies, provided pressured feedback, and reminded participants of time limits, or an autonomous style, where participants were encouraged to complete the puzzle logically and given supportive and nurturing feedback. No surprise, results interpretation revealed that motivation style affected cortisol production, with heightened cortisol being associated with the more stressful classroom situation. Thus, we can surmise that methodologies incorporating both behavioral outcomes and online physiological measures, such as hormonal secretions, provide a gateway to understand how biological markers of the SNS can indeed be useful predictors of future performance.
Principle #18—Displays of affiliation mimic parasympathetic nervous system activation
The quixotic power of love and kinship has engendered cultures and debilitated empires for over 4,000 years. Ranging from the 20-year quest of Menelaus in 1190 BCE avenging the abduction of his tempestuous wife Helen of Troy (which led to the Trojan Wars and the death of at least 150,000 people), to the promiscuous Cleopatra, who aided the collapse of the Roman Empire, to the abdication of King Edward VIII of England in 1948 to marry a commoner, the desire to be loved has immutably altered the course of history. The contentious view that love and platonic bonding conquers all is supported by some scholars of motivational science, who suggest that a primary motive of human existence is predicated upon fulfilling the need to feel connected to others (Gordon, Martin, Feldman, & Leckman, 2011; Ryan & Deci, 2000).
The motive of affiliation presumes that humans desire intimate physical and emotional connections to others that are selective, enduring, and consensual (Feldman, 2012). Historically, affiliative bonds with other individuals, groups, or communities were necessary for genetic propagation and human survival. Unified societies thrived, in part, because the constellation of affiliative group behaviors enhanced the probability of protection from threat and harm. Today, strong affiliative bonds are unnecessary for foraging food, fending off attacking animals, or appeasing disenchanted members of competing tribes (staff meetings excluded). However, the actualization of social attachment and the demonstration of pro-social behaviors are instrumental for overall psychological health. Confluent evidence from the disciplines of biology, psychology, and sociology reveal that the formation of early and enduring affiliative bonds is related to longevity, enhanced immunity to disease, and a lower incidence of psychological disorders (Puig, Englund, Simpson, & Collins, 2013). For our purposes, knowledge of the connection between biological and psychological indications of love and affiliation are useful to understand the purpose, process, and outcomes of performance behavior.
Recall that the parasympathetic nervous system is associated with regulating the body to a state of homeostasis, partially achieved through stress reduction. Physiologically, when the parasympathetic nervous system is activated, heart rate drops, blood pressure decreases, and erratic breathing is modulated. Behaviorally, stereotypic anxiety subsides as you relax and are overcome by an aura of contentment, relief, and satisfaction. A variety of recursive hormonal and autonomic neurological changes restore your body to pre-stress conditions. Now, evaluate the types of feelings you have when you are with a likeable partner, sibling, or friend, someone whose company you especially enjoy. Most people asked to describe bonding behaviors mention affectionate touch, interpersonal focus, and matched dyadic states related to feelings of calm, gratification, and safety (Schneiderman, Zagoory-Sharon, Leckman, & Feldman, 2012). You may have even experienced the phenomenon of temporary relief of cold or headache symptoms when you are with a certain special person. Logically, you may deduce similarities between behaviors associated with forming and maintaining platonic and romantic attachments, and parasympathetic nervous system activation. Feldman (2012) described this process of reciprocal integrated engagement and regulation of psychological and neurohormonal systems as “biobehavioral synchrony” (p. 381). The concordant biological and behavioral evidence concerning the need for affiliation reveals that changes in the parasympathetic nervous system and associated affiliative behavior are, indeed, coordinated and positively correlated (Schultheiss & Brunstein, 2010): Amorous feelings calm the brain.
The majority of studies that examine affiliative synchronicity investigate pair bonding, where attachments are formed between two individuals. Research most frequently examines mother–infant relations, although biopsychological evidence has now emerged revealing parallels to the formation of adult romantic and platonic bonds. The hormone Oxytocin OT is most frequently implicated as coinciding with affiliative behavior. Neurologically, OT transmission is part of the hypothalamic–pituitary–adrenal axis and related to dopaminergic neurons. When contextual conditions warrant the acceleration of bonding, such as when feeling safe and secure, parasympathetic efference increases prefrontal cortex activity, which concurrently accelerates OT–dopamine interactions, facilitating the motivation to bond (Feldman, 2012). Psychologically, individuals feel a sense of warmth, interpersonal trust, and security synchronized with corresponding increases in requited affection. Unique to OT transmission is the stability and reciprocity in hormonal activity between partners and even among triads (parental, romantic or filial, Feldman, 2012) whereby parallel transmissions result in mutually rewarding coordinated social behavior, such as reciprocated eye gaze, perception of closeness, feelings of mutual trust, and accurate detection of emotional states (Ross & Young, 2009). The effect of OT is so alluring that some researchers refer to OT as the “love drug” or the “cuddly chemical” (Dreu, Greer, Kleef, Shalvi, & Handgraaf, 2011).
Despite the purported benefits of OT, evidence for the universality of affiliative benefits is inconsistent. Specifically, certain populations (e.g., older women, Taylor, 2006) and those who experience loneliness or lack of social support (Bartz, Zaki, Bolger, & Ochsner, 2011) indicate decreased basal levels of OT despite affiliative behavior. These discrepancies suggest that OT levels may be stress indicators as well biological markers that orchestrate affiliation-seeking behavior in the quest to raise basal OT levels. In other words, the expectation of affiliation, not the affiliate bond per se, may be the underlying cause of OT transmissions.
Although elevated OT production is not the “love potion” described in some supplement advertisements and in the popular media (http://oxytocinspray.org), knowledge of the connection between affiliation motives and OT production is of practical value for at least three reasons. First, the results from dozens of studies suggest that OT production moderates social behavior (Ross & Young, 2009). I do not recommend offering learners or workers OT-laced brownies or advocate workplace socialization that interferes with policy or productivity; however, highly-functioning work teams and classrooms, as well as topics appropriate for constructivist learning, are naturalistic settings that can leverage the power of OT-affiliative synchronic bonds. Creating performance opportunities where collegial participants work together will likely jumpstart the OT pump, enhancing the probability of a coordinated work effort. Considering that group work requires the exchange of ideas and debating the relative merits of individual suggestions, we can presume that individuals will be more cooperative and open to suggestions when OT levels are elevated than when not. Empirical evidence in OT studies supports the hypothesis that individuals with strong affiliative motives exhibit measurable receptivity and foster group cohesiveness (Baumgartner, Heinrichs, Vonlanthen, Fischbacher & Fehr 2008; Gordon et al., 2011).
Second, although results from some studies are ambiguous (Theodoridou, Penton-Voak, & Rowe, 2013), frequently, individuals with higher basal OT levels are more perceptive of social and emotional cues (Domes, Heinrichs, Michel, Berger, & Herpertz, 2007; Guastella, Mitchell, & Dadds, 2008). These cues can range from understanding the meaning of nonverbal behavior to having more accurate perceptions of emotions of an individual when evaluating facial expression. The ability to detect feelings and emotions is critical considering nonverbal communication may frequently conflict with verbalizations, resulting in discrepant interpretation of messages (Argyle, 1988). Domes et al. (2007) used the phrase “affective mind reading” (p. 732) to describe the process of inferring dispositions and affect from physical cues emanating from the eye region alone, leading to the conclusion that high OT can predict more accurate inferences about affective states. These findings are especially useful to those individuals who may have difficulties interpreting socially relevant cues or who have sensory deficits, including children diagnosed with autism spectrum disorder (Bartz et al., 2010).
Third, higher basal OT levels are related to attachment and trust (Campbell, 2010). OT production, in many ways, mirrors the neurological pathways reminiscent of dopamine release in anticipation of monetary reward. The anticipation paradigm suggests that the expectation of reward is a powerful motivator correlated with positive mood and affect. This line of thought also hypothetically implies that high-OT individuals should be more receptive to creative problem solutions and more likely to take academic risks (e.g., answering questions in spite of the probability of being wrong) because of the higher degree of trust and comfort among constituent group members. Although many studies on the subject of reward anticipation use financial incentives (Foti & Hajcak, 2012), for others, social motivation alone is an equally powerful incentive to cultivate group performance. Regardless, the research on OT production leads the MD to understand that organizational trust is an integral factor in cultivating high performance in individuals and teams (Kramer & Lewicki, 2010).
In summary, cultivating OT production is not a panacea, nor is it a performance proxy suggesting that students or employees with high affiliation motive are stellar performers. Study results are ambiguous, and OT production is clearly mediated by a variety of contextual and biological factors, such as circadian rhythm, menstrual cycle, and interaction with the complex integrative human neural network (Campbell, 2010). In addition, some studies suggest that gaps in social relationships or anticipation of social harmony also catalyze OT production. However, any strategy that can replicate the contextual conditions associated with OT production will likely be organizationally advantageous and leverage the physiological corollaries of affiliation motives.
Principle #19—Achievement and incentive reward share similar neural response patterns
Most of us have personal strivings for success. We envision being the best at something, For example, you might strive to be the best parent, have the lowest golf score, or bake the best brownies. My friends Ron and Becky want to be known as the people who have the most nutcrackers on display in their home at holiday time! Whatever your preference, determining the target of our personal optimism involves a complex series of implicit and explicit estimations grounded in the process of setting goals. Sometimes the goals we set are motivated in comparison with others performing a similar task, such as selling more Girl Scout cookies than your neighbor does. Alternatively, we may strive to meet criteria of excellence, such as scoring above passing grade on a certification examination. Other times, we may focus on bettering our performance in comparison with our own prior results. Regardless of how goals are determined, individuals who are motivated by striving for excellence show a high need for achievement.
The need for achievement is well documented in the annals of motivation history. Pioneered by work in the mid-twentieth century, early views concerning achievement motivation were predicated upon a dichotomous philosophy espousing that individuals operated under two broad assumptions: they either approached tasks with the anticipation of success or avoided tasks when failure was expected (McClelland, Atkinson, Clark, & Lowell, 1976). This view suggested that positive emotions, such as excitement and pride, were associated with success, whereas negative consequences, including feelings of anxiety and shame, were related to failure. According to this polarized view, accomplished individuals would feel personally rewarded when reaching goals, whereas those unsuccessful would suffer frustration and potential humiliation because of the perception of failure. The behavioral cycle of success and failure closely aligns with how our neurological system responds to financial reward and incentive seeking, suggesting the interesting hypothesis that knowledge is actually a reward.
Empirical evidence for the biopsychological correlates of achievement is considerably more elusive than for other motives (Schultheiss & Brunstein, 2010). Studies generally reveal three systematic corollaries: increased dopamine levels in the brain, specific brain areas targeted, and hormonal release. Biologically, incentive seeking and reward activate the mesocorticolimbic system, focused in the ventral striatum, which comprises the nucleus accumbens, ventral caudate, and putamen (Knapp & Kornetsky, 2009). Collectively, these areas of the brain are one of the primary receptors of the neurotransmitter dopamine. The brain responds to the release of dopamine with feelings of confidence, serenity, and euphoric mood elevation. Although knowing the exact location of brain activation (referred to as localization) is important for neurologists, understanding motivation dictates that we also examine the behaviors that trigger the neuronal responses.
Most studies investigating achievement motivation control for motivational style or test for brain localization differences contingent upon completing certain tasks. McClelland (1995), in the first study of its kind, asked participants to complete mental math problems and found that high achievement motivation predicted better memory recall and decreased urination compared with a control group with low achievement motivation. McClelland speculated that since the hormone arginine vasopressin is an antidiuretic (substance that causes water retention), it was somehow linked to achievement motivation; however, this line of research surprisingly has remained relatively dormant since McClelland’s pioneering efforts to link physiology and a motivation for achievement.
Mizuno et al. (2008) used fMRI measures to compare the neural substrates activated by academic reward in comparison with those linked to monetary reward. Bilateral putamen activation was observed with strong localization similarities between those academically and financial motivated. Similarly, Lee, Reeve, Xue, and Xiong (2009) found unique, but overlapping, brain activations in the ventral striatum (where the putamen is located) for both financially motivated and inherently achievement-motivated individuals, leading to the conclusion that neural pathways of reward processing and episodic memory were shared. When varying incentives were offered during a pattern identification task, Taylor et al. (2004) observed similarities in neuronal activity between occasions when participants were offered a higher incentive compared with a lower one, and when participants activated executive control mechanisms of their working memory (highly related to achievement motivation). Finally, Kang et al. (2009) used a trivia task to investigate the relationships among intellectual curiosity, recall, and brain localization. Results analysis revealed that heightened curiosity (a proxy for achievement needs) predicted neuronal patterns similar to those in individuals anticipating reward. Answering trivia questions incorrectly was linked to higher putamen activation (the dopamine receptor), suggesting that the desire to learn new material may have a specific biological marker in comparison with the recollection of existing knowledge.
We can infer from these studies constancy in biological response patterns that align achievement motivation and reward paradigms in human laboratory subjects. Converting this knowledge to practice, we can confidently confirm the hypothesis that for many people, knowledge is rewarding. Thus, consideration of any activities that enhance knowledge would likely boost the engagement and productivity of individuals with achievement motivation, even if that motivation is not directly related to a particular task. We might also conclude that in well-defined conditions, extrinsic monetary reward may be a catalyst, inducing feelings of satisfaction and contentment based upon the biological corollary of dopamine release in the brain associated with reward anticipation. Of course, the extrinsic reward inference does not mean that focused attention or more efficient information processing can be artificially induced because not all individuals will have strong achievement motivation, and at times financial incentives can actually decrease interest and motivation (Lepper, Greene, & Nisbett, 1973). We should also remember that there is a general lack of consensus as to the exact neural mechanisms involved in regulating reward experience, and the information above shows that neural pathways of reward can be stimulated in multiple ways. The effects of reward will change within individuals over time and between tasks, thus cautious implementation of reward systems is essential.
Principle #20—Humanity is motivated to seek pleasure and avoid pain
A man hath no better thing under the sun than to eat, and to drink, and to be merry.
The Bible, Ecclesiastes 8:15
For there was never yet a philosopher that could endure the toothache patiently.
Shakespeare, Much Ado about Nothing
Under the guise of Shakespeare’s quill and God’s voice, much of life is dichotomized by a series of extremes: good versus evil, conservative versus liberal, controlled versus automatic, involved versus apathetic, hedonistic versus altruistic, Wilma versus Betty. These dualistic conceptions of human existence suggest that absolutes exemplify our beliefs and behaviors. In 1798, long before scholars scientifically investigated the nature of motivation, Jeremy Bentham espoused his utilitarian philosophy grounded in the dictum that all mankind seeks to maximize pleasure and minimize pain (Sober & Wilson, 1998). Bentham’s ideas illustrate psychological hedonism, a theory that closely resembles Principle #14 (p. 50), which indicates that humans are motivated to attain certain targets while avoiding others. Specifically, hedonistic theories of motivation contend that behaviorally and psychologically, all individuals are ultimately preordained to have an aversion to pain and a desire to actively pursue sensory pleasures. This explanation of motivation suggests that humans set goals and regulate behavior solely to satisfy hedonic needs: All else is subservient to hedonic pleasure, the singular focus of our daily efforts (Sober & Wilson, 1998).
Philosophically, few would debate that the prevailing goal of human existence, at least in part, is survival and a desire for all things subjectively satisfying. However, at least three conundrums discount the plausibility of hedonic behavior as the single force regulating the direction and intensity of our efforts. First, we pursue both ultimate goals, which are desired end-states, and instrumental goals that serve as incremental milestones to achieve our ultimate objectives (see pp. 155–157 for a detailed explanation of goal types). If we examine the chain of events that occurs between setting ultimate goals and enhancing the probability of reaching the goal, a personal willingness to endure pain (or at least discomfort and delay of gratification) is usually part of the formula. Examples abound, such as the “no pain, no gain” maxim associated with exercise; tolerating intellectually painful, but required, courses in order to earn a degree; or enduring the potential of conflicting motives associated with the seasonal visit to one’s in-laws as the sacrifice for a strong spousal relationship. Culturally desirable practices further illustrate that humans are willing to endure pain to enhance social standing and gain aesthetic pleasure. By example, 40% of the adult US population between the ages of 26 and 40 years pays someone to inject them with iron oxide and disazodiarylide, risking possible infection to flaunt permanently emblazoned body art, otherwise known as a tattoo (Pew Research Center, 2009). These examples suggest that although subjective pleasure or well-being are primary goals, considerable debate and entire books from eminent scholars argue that sensory pleasure alone does not dominate human behavior (Higgins, 2012).
Second, we must consider the subjective nature of pleasure and pain. By most accounts, pleasure is a conscious affective reaction to a stimulus that is perceived as biologically or psychologically rewarding for the organism (Kringelbach & Berridge, 2010). However, in order to be considered pleasurable, the stimulus must also be both liked and wanted. Although I like meatballs, I avoid meatballs when I wear my freshly starched white tuxedo shirt. For me, meatballs are a typically pleasing stimulus that becomes situationally aversive due to my cosmopolitan demeanor. I differ from my colleague Dr. Alex, who loves to eat sardines and wear t-shirts. For Alex, nothing beats the culinary delight of a hearty sardine and mustard sandwich on chewy Dreikernebrot bread while lounging in his favorite tattered Billy Bob’s Hideaway t-shirt. If your salivary reflex (which is an unconscious pleasure reaction) remains dormant, then you are likely nauseated by the taste or distinctive aroma of a freshly opened can of Norwegian sardines, suggesting that pleasure motivation varies according to individual. Hopefully, the scenario I just described is not so revolting that you begin to have feelings of pain, which according to some descriptions is defined as an unpleasant sensory experience. Pain, like pleasure, has motivational, affective, and neurological characteristics that are selectively interpreted by individuals (Guindon & Hohmann, 2009). Evaluation of pain usually coincides with conscious avoidance of the pain target, as the source of pain minimally has the potential for neural sensitivity or soft tissue damage. Most examples of environmentally induced pain are readily addressed by MDs (e.g., patching up injuries, regulating room temperature, and eliminating rancid odors, to name a few) and are not an interpretive concern here. However, subjective perceptions of interpersonal dynamics, organizational culture, or even responses to current events may become realistic interpretive nightmares due to the possibility of prompting a wide range of unpredictable cognitive and affective reactions associated with pain perception.
Third, we should ponder whether hedonic motivation is prompted by reaching the goal or by the process of goal pursuit. As Higgins (2012) suggested, we should avoid arbitrary conclusions that satisfaction of hedonic motivation is the cause of observed behavior. An obvious illustration is the satisfaction of hunger. Some of us indulge ourselves by ordering dessert after a large meal. Clearly, satisfying a specific bodily deficit is not the impetus for ordering a pie, with ice cream on the pie and syrup on the ice cream. Devoid of the physiological necessity for homeostatic restoration, we might surmise that ingestion is also a function of habit, social protocol, or perhaps the subjective positive valence associated with the process of consumption. Consider my neighbor Eugene, who spends hours casting his fishing line into our well-stocked bass pond but throws the fish back every time, apparently unconcerned about memorializing the goal! Those with amorous inclinations may also begrudgingly admit that the thrill of the romantic chase is more important than catching the fish (i.e., marrying your partner). Hopefully, these examples illustrate that hedonic motives are not exclusively satisfied by target attainment or successful avoidance. Instead, we should consider that irrespective of the target, behavioral–psychological reactions to the process of achieving goals are, at times, a sufficient motivating force to engage in the targeted behaviors. Fortunately, the culinary, haberdashery, and romantic dilemmas described, like many behavioral reactions to pleasurable or innocuous stimuli, have distinguishable neurobiological mechanisms both within and between individuals that assist motive identification (Berridge & Kringelbach, 2008).
Pleasure and pain can be measured in a variety of stereotypical ways, including facial expressions, pupil dilation, and orgasms (Kringelbach and Berridge, 2010). These obvious external indicators are a primary means of assessing the apparent affective state of an individual. The focal point is the façade, or what seems “apparent,” which may lead to inaccurate motivational inferences. Many times, superficial physiological indicators are unreliable indications of an individual’s underlying affect or cognitions. The unreliability of superficial measures dictates examination of a variety of “hedonic hotspots,” or nerve receptive centers in the brain to help enhance inference ability when evaluating hedonic behavior. Figure 3.2 illustrates the major hotspots and neural pathways in the brain that are activated when exposed to external stimuli that the individual perceives as potentially rewarding or debilitating. Similar to the biopsychological process of reward described earlier, neural receptors in the brain, such as the ventral palladium and nucleus accumbens, receive the neurotransmitter dopamine when stimulated during activities, such as eating, drinking, or sex. Dopamine release triggers a positive affective valence and the individual perceives these activities as pleasurable. Although these reactions are important, further elaboration on these pedestrian and carnal topics should not be of interest to the MD for a variety of ethical and statutory reasons.
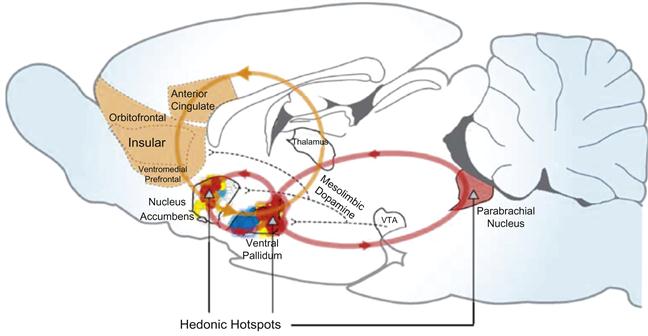
Examination of neurological research conducted in a number of common social settings reveals startling similarities to neural activation patterns observed when individuals are rewarded or punished. When studying the effects of deliberate exclusion from a cyber ball-tossing game, Eisenberger, Lieberman, and Williams (2003) used fMRI readings and observed greater activation of the anterior cingulate cortex (ACC, a pain hot spot) during game exclusion than during inclusion, which led to the conclusion that “social pain is analogous in its neurocognitive function to physical pain” (p. 292). Likewise, using fMRI measures, ACC activation was noted in bereaved women presented with familial grief-related stimuli picturing a mother or a sister, but activation in the nucleus accumbens (an affiliation reward center) was observed in women with self-reported stronger attachment to the departed individual (O’Connor et al., 2008). Individuals referring to themselves with negative self-referential cognitions, such as “I’m unlovable” or “no one desires me” activate biological consequences quite similar to those found in clinically depressed individuals (Slavich, O’Donovan, Epel, & Kemeny, 2010).
Conversely, activation of the striatum, which is usually observed during reward-related studies of satiety and thirst (see Principle #17, p. 57), was observed by Izuma, Saito, and Sadato (2008), who paired an individual’s positive personality feedback with an individual’s picture. Similarly, striatum neural activity is commonly observed when individuals believe they are treated fairly (Tabibnia, Satpute, & Lieberman, 2008), as well as when they are afforded the opportunity to give money to charitable causes (Izuma, Saito, & Sadato, 2010). Striatum activity is found during games of chance, usually when participants win. Curiously, similar striatum activity is found when some players lose, provided another’s loss is greater (Dvash, Gilam, Ben-Ze’ev, Hendler, & Shamay-Tsoory, 2010), perhaps suggesting that individuals take pleasure and are more motivated in defeat, but only when someone else suffers more!
A number of practical conclusions can be advanced when examining the positive and negative evidence associated with hedonic research. First, the subjective affective valence associated with external stimuli varies within and between individuals. People interpret similar stimuli through their own unique lens. What may be perceived as rewarding to one individual may be disdained by another. Further, individuals strive for a sense of optimal homeostatic balance (Leknes & Tracey, 2008), endeavoring to regulate emotional highs and lows. Although MDs will prosper by devoting substantial effort to determining what constituents perceive as pleasurable or noxious, generalization among individuals would likely be foolhardy. Awarding a turkey to a vegetarian or a box of chocolates to a fitness enthusiast may have null or unanticipated negative performance consequences.
Second, many organizations have deeply entrenched hierarchical structures that undermine who gets included and who does not. Appropriate inclusion criteria should be a concerted effort on the part of leadership, fostered by soliciting participants to volunteer when motivated to participate in a project. Excluding individuals without concrete justification based upon level or status alone may be perceived as the conscious infliction of emotional pain.
Third, neurological studies reveal dopamine production in anticipation of reward or a positive event. It seems prudent to avoid communicating unrealistic promises or false expectations to employees, who will easily become frustrated if expectations are not fulfilled.
Fourth, it seems that some individuals can be motivated by the expectation of pain as an instrumental goal. Individuals, such as athletes and dancers, who persevere through pain, intentionally orchestrate a positive posthedonic experience akin to a self-imposed and self-regulated reward. As Leknes and Tracey (2010) indicated, “let us not forget that enduring some discomfort is an efficient way of increasing pleasure and returning to homeostasis. Who has not tried fasting before a feast, or stayed in the sun until almost unbearably hot before jumping in the pool?” (p. 326). Consequently, it appears that for some, pain perceived as within one’s control may be judiciously used as a powerful motivator.
Principle #21—Motivated behavior is heritable and evolutionary
Are you feeling lazy? Tired of reading? Wishing this chapter was done? You can probably blame your parents, at least to a certain extent. Motivation, like hair color, height, and intelligence, has a specific heritable component. By heritable, I mean that behaviors, such as task orientation, the degree of conservatism you espouse, and even your spiritual commitment, are all determined, in part, by your unique genetic code. Of course, behaviors are also influenced by the environment, but to what extent? The field of behavior genetics reveals exactly what proportion of your disposition and behavior can be explained by the integrative role of genetics and environment. Mathematical heritability estimates measure the probable contribution of genetic factors as the source of a particular trait or behavior. These estimates are derived primarily from studies of monozygotic twins (identical) or from adopted children raised in similar or different environments. By comparing groups of identical twins raised in separate environments with genetically dissimilar groups of adopted siblings raised in the same environment, correlations are calculated that determine what percentage of a particular factor is estimated to be influenced by heredity. Although a host of methodological concerns are implicated in heritability calculations, statistical inferences from these types of studies provide valid data that suggest motivation has a heritable component. Table 3.2 shows selective heritability estimates, which, in general, indicate that 40–50% of the behavioral differences between individuals are explained by heredity (Plomin, 1990).
Table 3.2
Heritable estimates of selected motivational constructs
| Behavior | Heritable estimate (%) |
| Anxiety | 70 |
| Sociability | 64 |
| Dominance | 60 |
| Emotionality | 54 |
| Task orientation | 50 |
| Control | 44 |
| Aggression | 40 |
Once estimates of heritability are calculated, we then must consider the proportion of variability between individuals that is unexplained by genetics, in hopes of determining how environmental factors influence motivated behavior. The interpretation of heritable estimates should be guided by the concept of niche picking (Scarr & McCartney, 1983), which suggests that we are inclined to choose environments that leverage our inborn tendencies. Thus, if you are genetically predisposed with strong verbal ability, it is more likely that your parents will take you to the library than to a basketball court. Niche picking is empirically supported by the well-known phenomenon affecting reading motivation called the Matthew Effect (Stanovich, 1986). This effect suggests that skilled readers are deliberately afforded participation in more activities that promote reading comprehension, while their less advantaged peers tend to be relegated to learning contexts that do not support reading growth. Stanovich used the popular phrase “the rich get richer” to describe advantaged individuals who have the economic and social means to readily improve their existing talent and capitalize on existing strengths. In the absence of supportive contextual conditions, motivation to read is impeded, and the obstacles of the impoverished are intensified. Fortunately, the vicious cycle can be broken, and unfavorable genetic–environmental correlations can be “uncoupled” (Berk, 2008, p. 88). Uncoupling involves a concerted effort by caregivers to actively address and provide needed support. Several motivation variables are shown to be malleable to uncoupling interventions, including resilience (Kim-Cohen, Moffitt, Caspi, & Taylor, 2004), prosocial behavior (Knafo & Plomin, 2006), altruism (Krueger, Hicks, & McGue, 2001), and aggression (Moffitt, 2005). In aggregate, these studies confirm that despite genetic predisposition, proactive and intentional strategies can overcome the negative influence of latent genetic–environmental risks (Moffitt, 2005).
The conclusion that innate predispositions can be mediated through defined actions provides interesting fodder to contemplate the evolutionary nature of motivated behavior. Although the empirical evidence supporting the heritability of motivation is strong, due to the current methodological restriction for time travel, little, if any, empirical data can inscrutably verify that motivated behavior has historical variation. However, a variety of evidence at the cellular and behavioral levels suggests that humans functionally change when a particular strategy is determined to be useful and beneficial. A premise of evolutionary theory is that human characteristics evolve as a means to help the organism survive and reproduce and variants in efficacious behavior are passed along to future generations at a greater frequency than those strategies shown to be less beneficial (Sober & Wilson, 1998). Scholars debate the source and type of psychological adaptation (Confer et al., 2010), but clearly at minimum, survival and mating strategies have an evolutional trajectory (Buss, 2005). Nairne, Thompson, and Pandeirada (2007) used variations in memory priming tasks and found that participants had a higher probability of recalling words related to survival mechanisms (e.g., remembering landmarks, constellations, weather patterns, and food words) than words with no survival connotation. Additionally, a host of other strategy-based interventions have concluded that evolutionary-based preferences exist for some behaviors, such as how females choose a mating partner, how groups deal with performance laggards, and how female superiority with regard to spatial skills has evolved (Confer et al., 2010). Collectively, these studies suggest that domain-specific adaptation in behaviors is influenced by survival motivation.
Additionally, albeit likely of far less interest to the MD, developmental neuroscience provides conclusive evidence to support neuronal plasticity (i.e., molecular changes to existing mechanisms at the cellular level); that is, as organisms, both animals and humans adapt to changes in their respective living environments. Behaviors as fundamental as a baby crying because of separation anxiety, as seen in experiments where rat pups are involuntarily removed from the mother, are routinely studied at both the cellular and behavioral levels. Rat mothers show measurable changes in neurotransmitter receptors in the brain when involuntarily separated from their pups. Corresponding behavioral reactions of heightened anxiety ensue (Hofer, 2009). In other words, evolved changes in the detection system of the rat mother can be observed at both genetic and behavioral levels, with each mechanism showing graded responses over time. Parallel regulatory mechanisms exist in humans, suggesting that as successive generations undergo behavior adaptations, measurable genetic changes should also be expected.
Although conclusive evidence supporting the evolutionary nature of behavior continues to emerge at the human level, we can reliably conclude that at the very least, motivated behavior is determined in multiple ways (Cacioppo & Berntson, 2009). As MDs, acute awareness of the behavioral manifestations of human motives found in the classroom or workplace can be positively or negatively associated with biological markers. Research in humans that use techniques, such as event-related potentials, that measure brain voltage have been shown to discriminate changes undetectable by behavioral observation alone (Mills et al., 2013). Multiple diagnostic approaches enhance the ability to decipher relevant evidence from what Confer et al. (2010) referred to as “noise” (p. 110), which happens when unique combinations of biological and contextual factors result in random unpredictable behaviors, potentially leading to false inferences. Clearly, the consideration and realization that unitary behavioral evidence is insufficient, yet necessary, to advance conclusions will serve the aspiring student of motivational science well.
Chapter summary/conclusions
Faced with a myriad of evidence that requires constant vigilance to determine situational relevance, the MD is bombarded with seemingly isomorphic behaviors. The challenge of deciphering the exact integrative nature of the behaviors and to what extent the behaviors can reliably yield conclusions about an individual is quite puzzling. The first step in the process of mediation is the realization that individual perceptions are most likely selective and arbitrary interpretations of stimuli that may significantly vary both within and between individuals.
Although behavioral observation alone can be misleading and potentially lead to spurious interpretations, individuals will be hard pressed to control or disguise the representations of their biological markers. Accurate detection of biophysical evidence, in many cases, will provide the gateway to affirmation for the practitioner. Nervous system and corresponding hormonal activity are predictable, albeit challenging to observe. Pacing the hallways with fMRI equipment or thermodynamic instruments is impractical for most; however, the concomitant physiological accompaniments of behavior can be readily observed, as individuals routinely display repetitive response patterns to people and events. Evidence of neurological activity, such as pupil dilation, breathing patterns, and facial expressions, can be easily detected and should be a routine part of the MD’s diagnostic process when contemplating the source of motivations.
The current state of biopsychology research presents two main challenges. First, new findings occur regularly and require active practitioner diligence to remain current. Second, biopsychological findings are frequently ambivalent, creating interpretive difficulties. In spite of these concerns, the evidence presented in the current chapter should clearly add to the toolbox of the MD. Regardless, identification of confluent patterns of behavior and biology together will provide more robust and reliable interpretive data than either source in isolation.
Next steps
The current chapter has illuminated a number of findings that generate a foundation of knowledge concerning how human neuronal and hormonal mechanisms interact with certain behaviors. Clear links between biology and motivation are supported by empirical evidence, but how does motivation change over time? As individuals grow and mature, biological change is inevitable. Additionally, as individuals interact with their environments, they undergo a series of psychological changes based upon reactions to their collective experience. The experience, in turn, influences motives, which, like many aspects of human existence, have a defined developmental trajectory that shapes the goals we set, the strategies we use, and the outcomes we attain. As an example to help understand the trajectory of motivation, we will examine the maturation of two siblings Cheryl and Rebecca Hines, who, at face value, appear to be drastically different: One is a famous actress and the other a prominent college professor. We next turn to how motivation changes over the course of the lifespan and discuss critical periods of change that influence how we choose to navigate our lifelong motivational journey.
End of chapter motivational minute
Principles covered in this chapter:
13. Neurological/endocrinological evidence informs or refutes behavioral evidence—many behaviors have predictable physiological corollaries. Analysis of behavior and biological markers together typically yields more accurate inferences than analysis of behaviors alone.
14. Neurological/endocrinological inferences are multi-dimensional—a complex interaction exists between observed behavior and human physiology. Evidence can be interpreted at the genetic, synaptic, autonomic, skeletal, cognitive, or systems levels, unitarily or in combination.
15. The brain is a perceptual filter that influences subjective reality—humans process information through the limitations of their perceptual and belief networks. Similar stimuli can evoke multiple responses depending upon context and interpretive variables.
16. Neurological system organization facilitates or inhibits action—the body strives to maintain a comfortable state of homeostasis. Reactions to behaviors, routinely exhibited in practice, mirror biological triggers found in the CNS.
17. Power and social dominance displays mimic sympathetic nervous system activation—when individuals make decisions, control, or take actions that determine the behaviors of others the biological response patterns resemble an organism that is primed for flight or fight.
18. Displays of affiliation mimic parasympathetic nervous system activation—when individuals exhibit bonding behavior, the physiological corollaries resemble an organism in a state of homeostasis, marked by stress reduction and OT production.
19. Achievement and incentive reward share similar neural response patterns—the biological responses associated with achievement motivation closely align with how our neurological system responds to financial reward. Contextual conditions that promote inclusion and involvement can positively motivate individuals.
20. Humanity is motivated to seek pleasure and avoid pain—humans pursue ultimate and instrumental goals. Hedonistic theories of motivation contend that, ultimately, all human motivations are based upon attaining pleasure and avoiding pain.
21. Motivated behavior is heritable and evolutionary—substantial evidence supports the view that motivation has a heritable component. Emerging evidence in the field of evolutionary psychology confirms that motives undergo change at the genetic level.
Key terminology (in order of chapter presentation):
 Neuroanatomy—the study of human neurological and hormonal systems.
Neuroanatomy—the study of human neurological and hormonal systems.
 Dualism—a philosophical orientation which asserts that human behavior is explained by two distinct and ontologically separate governing entities.
Dualism—a philosophical orientation which asserts that human behavior is explained by two distinct and ontologically separate governing entities.
 Spurious—the process of attributing causality to an unwarranted cause when examining the relationship between two or more variables.
Spurious—the process of attributing causality to an unwarranted cause when examining the relationship between two or more variables.
 Ambiguous temporal precedence—the uncertainty related to determining the casual path of variables. By example, it is unknown if physiological reactions precede a behavior, or if initiation of a behavior precedes the physiological response.
Ambiguous temporal precedence—the uncertainty related to determining the casual path of variables. By example, it is unknown if physiological reactions precede a behavior, or if initiation of a behavior precedes the physiological response.
 Localization—the process of pinpointing specific areas of the human anatomy responsible for particular hormonal or neurological functioning.
Localization—the process of pinpointing specific areas of the human anatomy responsible for particular hormonal or neurological functioning.
 Plasticity—the ability of human physiological architecture to undergo changes in neuronal and cellular composition based upon experience.
Plasticity—the ability of human physiological architecture to undergo changes in neuronal and cellular composition based upon experience.


 Alexis (Alex) Paige Dixon contracted pneumonia halfway through fourth grade. Prior to that, she was a bright, kind, and sometimes socially awkward child. Academics came easily to her. She loved animals, playing the piano, art, and being outside. The pneumonia triggered a glitch in her central nervous system (CNS), and she would never fully recover from her illness. The glitch caused her body to contort, and she experienced considerable pain. She missed a great deal of school and spent many of her days in hospitals seeking a cure for her illness. By the time she began sixth grade, she was painfully confined to a wheelchair.
Alexis (Alex) Paige Dixon contracted pneumonia halfway through fourth grade. Prior to that, she was a bright, kind, and sometimes socially awkward child. Academics came easily to her. She loved animals, playing the piano, art, and being outside. The pneumonia triggered a glitch in her central nervous system (CNS), and she would never fully recover from her illness. The glitch caused her body to contort, and she experienced considerable pain. She missed a great deal of school and spent many of her days in hospitals seeking a cure for her illness. By the time she began sixth grade, she was painfully confined to a wheelchair.