CHAPTER 8
Monoamine Oxidase Inhibitors
K. Ranga Rama Krishnan, M.D.
History and Discovery
Monoamine oxidase inhibitors (MAOIs) were first identified as effective antidepressants in the late 1950s. An early report suggested that iproniazid, an antitubercular agent, had mood-elevating properties in patients who had been treated for tuberculosis (Bloch et al. 1954). Following these observations, two studies confirmed that iproniazid did indeed have antidepressant properties (Crane 1957; Kline 1958). Zeller (1963) showed that iproniazid caused potent inhibition of monoamine oxidase (MAO) enzymes both in vivo and in vitro in the brain. He also reported that the medication reversed some of the actions of reserpine. Because reserpine produced significant depression as a side effect, it was suggested that iproniazid might have mood-elevating properties.
The use of iproniazid soon fell into disfavor because of its significant hepatotoxicity. Other MAOIs, both hydrazine derivatives (e.g., isocarboxazid, phenylhydrazine) and nonhydrazine derivatives (e.g., tranylcypromine), were introduced. These MAOIs were not specific for any subtype of MAO enzyme, and they were irreversible inhibitors of MAO (see next section, “Monoamine Oxidase”). Their use has been rather limited because hypertensive crisis by the MAOIs may occur in some patients from potentiation of the pressor effects of amines (such as tyramine) in food (Blackwell et al. 1967).
In more recent years, there has been a resurgence of interest in the development of new MAOIs—that is, in development of MAOIs that are more selective for specific subtypes of MAO enzyme and that are reversible in nature. Reversible monoamine oxidase A (MAO-A) inhibitors, such as moclobemide, have been introduced in Europe but are not available in the United States. Newer MAOIs, such as selegiline, a monoamine oxidase B (MAO-B) inhibitor, have been introduced. Table 8–1 classifies the MAOIs by structure, selectivity, and reversibility.
Drug |
Hydrazine |
Selective |
Reversible |
Phenelzine |
Yes |
No |
No |
Isocarboxazid |
Yes |
No |
No |
Tranylcypromine |
No |
No |
No |
Selegiline |
No |
No |
|
Clorgylinec |
No |
Yesd |
No |
Moclobemidee |
No |
Yesd |
Yes |
Brofarominec |
No |
Yesd |
Yes |
aSelective for monoamine oxidase B (MAO-B) at lower doses. bBecomes nonselective at higher doses. cNever marketed in the United States. dSelective for monoamine oxidase A (MAO-A). eNot commercially available in the United States. |
|||
Monoamine Oxidase
A and B Isoenzymes
MAO is widely distributed in mammals. Two isoenzymes, MAO-A and MAO-B, are of special interest (Cesura and Pletscher 1992). Both are present in the central nervous system (CNS) and in some peripheral organs. For example, MAO-A is present in the liver, heart, and pancreas, and MAO-B is present in the liver, posterior pituitary, renal tubules, and endocrine pancreas (Saura et al. 1992). Both MAO-A and MAO-B are present in discrete cell populations within the CNS. MAO-A is present in both dopamine (DA) and norepinephrine (NE) neurons, whereas MAO-B is present to a greater extent in serotonin (5-HT)–containing neurons. Both are also present in nonaminergic neurons in various subcortical regions of the brain. Glial cells also express MAO-A and MAO-B (Cesura and Pletscher 1992).
The physiological functions of these two isoenzymes have not been fully elucidated. The main substrates for MAO-A are epinephrine, NE, and 5-HT. The main substrates for MAO-B are phenylethylamine, phenylethanolamine, tyramine, and benzylamine. DA and tryptamine are metabolized by both isoenzymes. The localization of the MAO subtypes does not fully correspond to the neurons containing the substrates. The reason for this discrepancy is unknown. The occurrence of the MAO-B form in 5-HT neurons may actually protect these neurons from amines (other than 5-HT) that could be toxic to them (Cesura and Pletscher 1992).
The primary structures of MAO-A and MAO-B have been fully described. MAO-A has 527 amino acids, and MAO-B has 520 amino acids. About 70% of the amino acid sequence of the two forms is homologous. The genes for both isoenzymes are located on the short arm of the human X chromosome. MAO-A and MAO-B are linked and have been located in the XP11.23–P11 and XP22.1 regions, respectively. The genes span about 70 kilobases (kb) and consist of about 15 exons and 14 introns. MAO-A has two messenger RNA (mRNA) transcripts of 2.1 and 5.0 kb in length. MAO-B has a 3-kb mRNA single transcript (Cesura and Pletscher 1992). A rare inherited disorder, Norrie’s disease, is characterized by deletion of both genes; patients with this disorder have very severe mental retardation and blindness. Another rare inherited disorder is Brunner syndrome, caused by a mutation in the MAO-A gene. It is characterized by impulsive aggressiveness and mild mental retardation (Brunner et al. 1993).
The subunit composition of MAO is unknown. The enzyme is primarily found in the outer mitochondrial membrane; flavin adenine dinucleotide is a cofactor for both MAO-A and MAO-B.
Because the cofactor domain is the same for both of the MAO isoenzymes, the structural differences responsible for substrate specificity are believed to lie in regions of the protein that bind to the hydrophobic moiety of the substrate. Although DA is considered to be a mixed substrate for both MAO-A and MAO-B, the breakdown of DA in the striatal regions of the brain is preferentially by MAO-B. In other regions, MAO-A may be more important. There may be regional differences as to which isoenzyme is responsible for the metabolism of other biogenic amines that are substrates for both forms of MAO (Cesura and Pletscher 1992).
Enzyme Kinetics
The enzyme kinetics of MAO-A have not been well studied. The enzyme kinetics for MAO-B, for which more information is available, depend on the nature of the substrate. Some substrates (e.g., tyramine) go through ping-pong mechanisms characterized by oxidation of the amine to the imine form, which is subsequently released from the reduced enzyme before reoxidation occurs. Other substrates (e.g., benzylamine) involve formation of a tertiary complex with the enzyme and oxygen (Husain et al. 1982; Pearce and Roth 1985; Ramsay and Singer 1991).
Positron Emission Tomographic Studies of MAO-A in Psychiatric Disorders
[11C]Harmine is a selective reversible positron emission tomography (PET) radiotracer with high brain uptake that binds with high affinity to MAO-A. A study using this tracer reported highly significant elevations in brain MAO-A binding during episodes of major depressive disorder that persisted even after selective serotonin reuptake inhibitor (SSRI) treatment (Meyer et al. 2009). Interestingly, subjects with higher MAO-A levels had a higher rate of major depressive episode recurrence (Meyer et al. 2009).
Mechanism of Action
The target function of MAOIs is regulation of the monoamine content within the nervous system. Because MAO is bound to the outer surface of the plasma membrane of the mitochondria, in neurons MAO is unable to deaminate amines that are present inside stored vesicles and can metabolize only amines that are present in the cytoplasm. As a result, MAO maintains a low cytoplasmic concentration of amines within the cells. Inhibition of neuronal MAO produces an increase in the amine content in the cytoplasm. Initially, it was believed that the therapeutic action of MAOIs was a result of this amine accumulation (Finberg and Youdim 1984; Murphy et al. 1984, 1987). More recently, it has been suggested that secondary adaptive mechanisms may be important for the antidepressant action of these agents.
After several weeks of treatment, MAOIs produce effects such as a reduction in the number of β-adrenoceptors, α1- and α2-adrenoreceptors, and serotonin type 1 (5-HT1) and serotonin type 2 (5-HT2) receptors. These changes are similar to those produced by the chronic use of tricyclic antidepressants (TCAs) and other antidepressant treatment (DaPrada et al. 1984, 1989).
MAOIs can be subdivided on the basis of not only the particular type of enzyme inhibition but also the type of inhibition they produce (reversible or irreversible). The reversible MAOIs are basically chemically inert substrate analogs. MAOIs are recognized as substrates by the enzyme and are converted into intermediates by the normal mechanism. These converted compounds react to the inactive site of the enzyme and form a stable bound enzyme. This effect occurs gradually, and there is usually a correlation between the plasma concentration of the reversible inhibitors and pharmacological action.
Pharmacological Profile
The classic MAOIs inhibit both forms of the enzyme and are divided into two main subtypes: hydrazine and nonhydrazine derivatives (Figure 8–1). The hydrazine derivatives, two of which are currently available (phenelzine and isocarboxazid), are related to iproniazid. One nonhydrazine derivative, tranylcypromine, is commercially available.
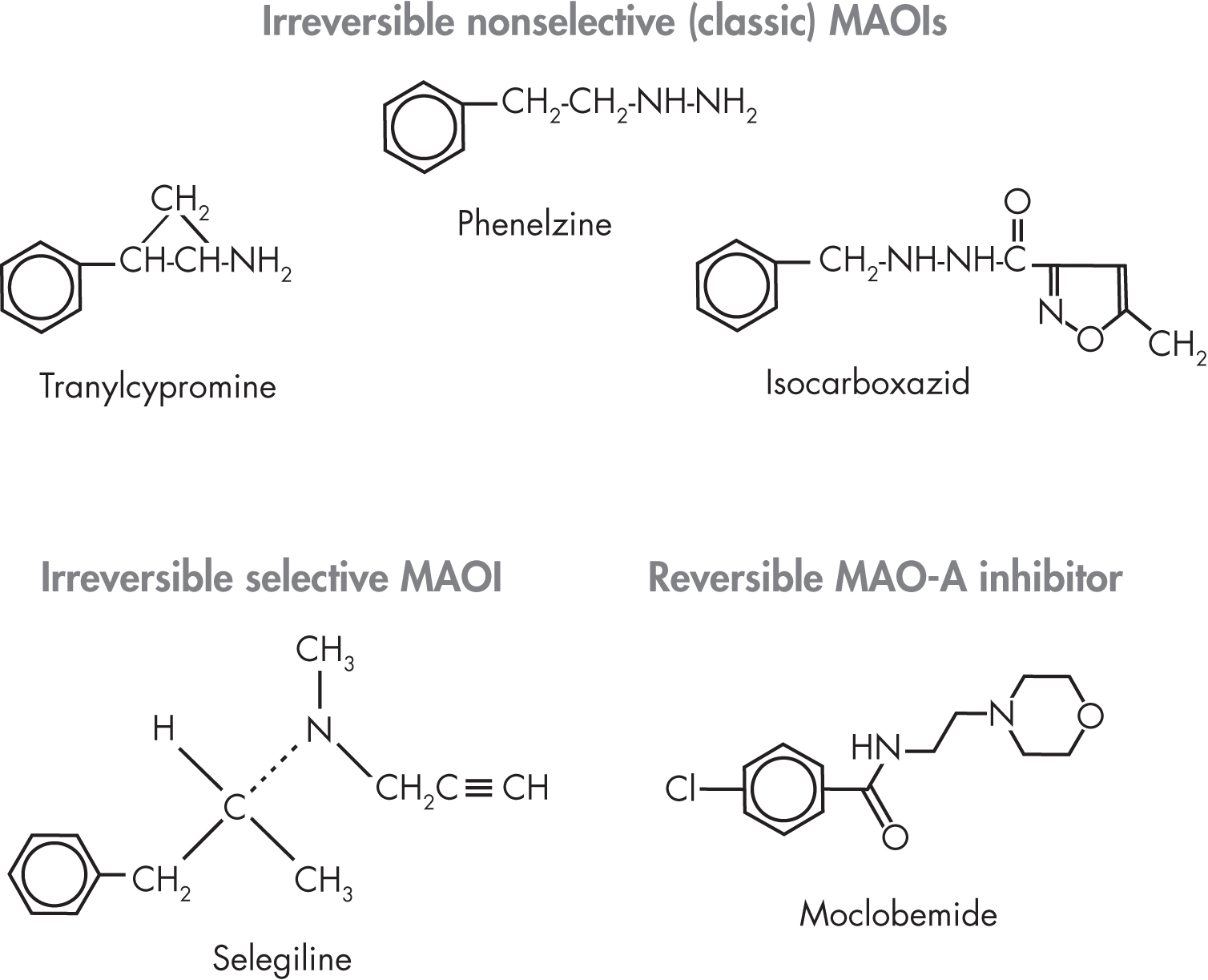
FIGURE 8–1. Chemical structures of monoamine oxidase inhibitors (MAOIs).
Among the selective MAOIs, clorgyline (which was never marketed in the United States) is an example of an irreversible inhibitor of MAO-A, whereas selegiline is an irreversible inhibitor of MAO-B. Moclobemide is the only reversible inhibitor of MAO-A on the market (available in the United Kingdom and Australia, but not in the United States).
Three classic MAOIs (i.e., phenelzine, isocarboxazid, and tranylcypromine) are of clinical interest. Clinicians must recognize that these drugs not only inhibit MAO but also exert other actions that may be clinically relevant. Thus, these compounds can block MAO uptake—tranylcypromine more than isocarboxazid or phenelzine. In addition, because tranylcypromine is structurally similar to amphetamine, it is believed to exert stimulant-like actions in the brain. Many issues are common to all three of these MAOIs.
Irreversible MAOIs
Indications and Efficacy
As discussed in the following subsections, irreversible MAOIs have been found to be effective in treating a variety of psychiatric disorders (Table 8–2).
Definitely effective |
Other possible uses |
Atypical depression |
Obsessive-compulsive disorder |
Major depressive disorder |
Narcolepsy |
Dysthymia |
Headache |
Melancholia |
Chronic pain syndrome |
Panic disorder |
Generalized anxiety disorder |
Posttraumatic stress disorder |
Premenstrual dysphoria |
Bulimia nervosa |
|
Atypical facial pain |
|
Anergic depression |
|
Treatment-resistant depression |
|
Parkinson’s diseasea |
|
aSelegiline is the only MAOI that is useful in the treatment of Parkinson’s disease. |
|
Major Depressive Disorder and Atypical Depression
Many studies have examined the efficacy of the irreversible MAOIs in the treatment of different types of depression. MAOIs have been effective in the treatment of major depressive disorder and atypical depression (defined as depression with anxiety or chronic pain, reversed vegetative symptoms, and rejection sensitivity [Quitkin et al. 1990]) (Davidson et al. 1987a; Himmelhoch et al. 1982, 1991; Johnstone 1975; Johnstone and Marsh 1973; McGrath et al. 1986; Paykel et al. 1982; Quitkin et al. 1979, 1990, 1991; Rowan et al. 1981; Thase et al. 1992; Vallejo et al. 1987; White et al. 1984; Zisook et al. 1985). Although early studies of relatively low-dosage regimens suggested that the efficacy of MAOIs was lower than that of TCAs, more recent studies have documented that their efficacy is comparable.
Quitkin et al. (1979, 1991) reviewed both phenelzine and tranylcypromine studies in patients with either atypical depression or melancholic depression. The authors reported that phenelzine appeared to be effective for the treatment of atypical depression.
Relatively few studies have investigated MAOIs in patients with endogenous depression (i.e., depression without known precipitating factors). From the limited number of patient studies, it is difficult to conclude that phenelzine is effective in the treatment of these patients. In addition, very few well-controlled studies have compared tranylcypromine with placebo. Three of the four studies that compared tranylcypromine with placebo showed that tranylcypromine was more effective (Himmelhoch et al. 1982; Moises and Beckmann 1981; White et al. 1984). In one study, a nonsignificant trend was found favoring tranylcypromine (Nolen 1989). Studies have also documented the efficacy of tranylcypromine in treating anergic depression and, at high doses, treatment-resistant depression (Himmelhoch et al. 1982, 1991; Thase et al. 1992; White et al. 1984). In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, patients who had failed to respond to at least three treatment options were randomly assigned to tranylcypromine or a combination of venlafaxine and mirtazapine. Remission rates were modest for both the tranylcypromine group and the extended-release venlafaxine plus mirtazapine group, and the rates were not statistically different between groups (McGrath et al. 2006).
The heterogeneity of acetylation rate may account for some of the variance in response to phenelzine (Johnstone 1975; Johnstone and Marsh 1973; Paykel et al. 1982; Rowan et al. 1981). One-half of the patients in a given population are often slow acetylators. An initial study by Johnstone and Marsh (1973) suggested that slow acetylators improve more with phenelzine than do fast acetylators. Other groups have been unable to confirm the relation between acetylation, acetylator type, and response to MAOIs.
Early studies suggested that irreversible MAOIs are particularly effective in patients who have atypical depression. The concept of atypical depression remains controversial and has not been completely validated. In general, patients with atypical depression have an earlier age at onset than do patients with melancholic depression, and the prevalence of dysthymia, alcohol abuse, sociopathy, and atypical depression is increased in the relatives of patients with atypical depression. The best differentiating criterion appears to be that phenelzine and other irreversible MAOIs are more effective than TCAs in treating these patients (Cesura and Pletscher 1992; Quitkin et al. 1990; Zisook et al. 1985). A meta-review noted evidence that MAOIs are superior to TCAs, but not SSRIs, in treating atypical depression (Cipriani et al. 2007).
Some studies have also suggested that MAOIs are effective in treating major depressive disorder and melancholic depression (Davidson et al. 1987a; McGrath et al. 1986; Vallejo et al. 1987). In a Cochrane review, Lima and Moncrieff (2000) noted that MAOIs were comparable in efficacy to other classes of antidepressants in treating dysthymia.
Panic Disorder
Both single- and double-blind studies have demonstrated the efficacy of phenelzine and iproniazid in treating panic disorder (Lydiard et al. 1989; Quitkin et al. 1990; Tyrer et al. 1973). About 50%–60% of patients with panic disorder respond to irreversible MAOIs. In the early stages of treatment, patients may have a worsening of symptoms. This is reduced in clinical practice by combining the MAOI with a benzodiazepine for the initial phase of the study. It has been suggested that in addition to having an antipanic effect, phenelzine has an antiphobic action (Kelly et al. 1971). The time course of effect and the dose used are similar to those for major depressive disorder.
Social Phobia
Liebowitz et al. (1992) reported that phenelzine is effective in treating social phobia. In an open-label study, Versiani et al. (1988) suggested that tranylcypromine is effective. Versiani et al. (1992) also demonstrated the efficacy of the reversible MAOI moclobemide in a double-blind study. About 50% of patients respond to MAOIs, and the onset of response is gradual (usually about 2–3 weeks) (Liebowitz et al. 1992).
A Cochrane review of pharmacotherapy for social phobia noted that whereas classic irreversible MAOIs were comparable in efficacy to SSRIs, reversible MAOIs were less efficacious (Stein et al. 2004).
Obsessive-Compulsive Disorder
Although initial case reports suggested that irreversible MAOIs may be effective in treating obsessive-compulsive disorder (Jenike 1981), no double-blind studies conducted have indicated efficacy (Jenike et al. 1997).
Posttraumatic Stress Disorder
The classic MAOI phenelzine has been proven effective for the treatment of posttraumatic stress disorder (PTSD) in single-blind trials (Davidson et al. 1987b) and a double-blind crossover trial (Kosten et al. 1991).
Generalized Anxiety Disorder
MAOIs are not usually used to treat generalized anxiety disorder because the risk–benefit ratio favors the use of SSRIs, azapirones, or benzodiazepines. When they are used, MAOIs are used primarily for treating treatment-resistant generalized anxiety disorder.
Bulimia Nervosa
Both phenelzine and isocarboxazid have been shown to be effective in treating some symptoms of bulimia nervosa (Kennedy et al. 1988; McElroy et al. 1989; Walsh et al. 1985, 1987).
Premenstrual Dysphoria
Preliminary studies and clinical experience suggest that MAOIs may be effective in the treatment of premenstrual dysphoria (Glick et al. 1991).
Chronic Pain
MAOIs are believed to be effective in the treatment of atypical facial pain and other chronic pain syndromes. However, only limited data on these conditions are available.
Neurological Diseases
The classic MAOIs have not been found to be effective for treating neurological disorders such as Parkinson’s disease and Alzheimer’s dementia. However, the MAO-B inhibitor selegiline has been shown to be effective in slowing the progression of Parkinson’s disease (Cesura and Pletscher 1992), although the mechanism underlying this effect is unknown.
Side Effects and Toxicology
The side effects of irreversible MAOIs are generally not more severe or frequent than those of other antidepressants (Zisook 1984). The most frequent side effects include dizziness, headache, dry mouth, insomnia, constipation, blurred vision, nausea, peripheral edema, forgetfulness, fainting spells, trauma, urinary hesitancy, weakness, and myoclonic jerks. Loss of weight and appetite may occur with isocarboxazid use (Davidson and Turnbull 1982). Hepatotoxicity is rare with the currently available irreversible MAOIs, unlike with iproniazid. However, liver enzymes, such as aspartate transaminase and alanine transaminase, are elevated in 3%–5% of patients. Liver function tests must be done when patients have symptoms such as malaise, jaundice, and excessive fatigue.
Some side effects of irreversible MAOIs first emerge during maintenance treatment (Evans et al. 1982). These side effects include weight gain (which occurs in almost one-half of patients), edema, muscle cramps, carbohydrate craving, sexual dysfunction (usually anorgasmia), pyridoxine deficiency (Goodheart et al. 1991), hypoglycemia, hypomania, urinary retention, and disorientation. Peripheral neuropathy (Goodheart et al. 1991) and speech blockage (Goldstein and Goldberg 1986) are rare side effects of irreversible MAOIs. Weight gain is more of a problem with hydrazine compounds, such as phenelzine, than with tranylcypromine. Therefore, weight gain that is caused by hydrazine derivatives is an indication to switch to tranylcypromine. Edema is also more common with phenelzine than with tranylcypromine.
The management of some of these side effects can be difficult. Orthostatic hypotension is common with irreversible MAOIs. Addition of salt and salt-retaining steroids such as fludrocortisone (9-α-fluorohydrocortisone) is sometimes effective in treating orthostatic hypotension. Elastic support stockings are also helpful. Small amounts of coffee or tea taken during the day also keep the blood pressure elevated. The dose of fluorohydrocortisone should be adjusted carefully, because in elderly patients it could provoke cardiac failure resulting from fluid retention.
Sexual dysfunction that occurs with irreversible MAOIs is also difficult to treat. Common problems include anorgasmia, decreased libido, impotence, and delayed ejaculation (Harrison et al. 1985; Jacobson 1987). Cyproheptadine is sometimes effective in treating sexual dysfunction such as anorgasmia. Bethanechol may also be effective in some patients.
Insomnia occasionally occurs as an intermediate or late side effect of irreversible MAOIs. Changing the time of administration does not seem to help much, although dosage reduction may be helpful. Adding trazodone at bedtime is effective, but this should be done with caution. Myoclonic jerks, peripheral neuropathy, and paresthesias, when present, are also difficult to treat. When a patient has paresthesia, the clinician should evaluate for peripheral neuropathy and pyridoxine deficiency. In general, patients taking irreversible MAOIs should also receive concomitant pyridoxine therapy. When myoclonic jerks occur, patients can be treated with cyproheptadine.
Irreversible MAOIs also have the potential to suppress anginal pain; therefore, coronary artery disease could be overlooked or underestimated.
Patients with hyperthyroidism are more sensitive to irreversible MAOIs because of their overall sensitivity to pressor amines. Irreversible MAOIs can also worsen hypoglycemia in patients taking hypoglycemic agents such as insulin.
Dietary Interactions
After the introduction of irreversible MAOIs, several reports of severe headaches in patients who were taking these compounds were published (“Cheese and Tranylcypromine” 1970; Cronin 1965; Hedberg et al. 1966; Simpson and Gratz 1992). These headaches were caused by a drug–food interaction. The risk of such an interaction is highest for tranylcypromine and lower for phenelzine, provided that the dose of the latter remains low. However, the clinician must keep in mind that this interaction can occur even at low doses with any of the classic MAOIs. The interaction of irreversible MAOIs with food has been attributed to increased tyramine levels. Tyramine, which has a pressor action, is present in a number of foodstuffs. It is normally broken down by the MAO enzymes and has both direct and indirect sympathomimetic actions. It has been suggested that the potentiation of tyramine by an MAOI may be secondary to increased release of NE rather than to the MAOI. Adrenaline would increase the indirect sympathetic activity of tyramine. The spontaneous occurrence of hypertensive crises in a few patients lends support to this hypothesis (O’Brien et al. 1992; Zajecka and Fawcett 1991).
The tyramine effect of food is potentiated by MAOIs 10- to 20-fold. A mild tyramine interaction occurs with about 6 mg of tyramine; 10 mg can produce a moderate episode, and 25 mg can produce a severe episode that is characterized by hypertension, occipital headache, palpitations, nausea, vomiting, apprehension, occasional chills, sweating, and restlessness. On examination, neck stiffness, pallor, mild pyrexia, dilated pupils, and motor agitation may be seen. The reaction usually develops within 20–60 minutes after ingestion of food. Occasionally, the reaction can be very severe and may lead to alteration of consciousness, hyperpyrexia, cerebral hemorrhage, and death. Death is exceedingly rare (Cooper 1989).
The classic treatment of the hypertensive reaction is phentolamine (5 mg) administered intravenously (Youdim et al. 1987; Zisook 1984). Nifedipine, a calcium channel blocker, has been shown to be effective (Stumpf 1988). Nifedipine has an onset of action of about 5 minutes, and it lasts approximately 3–5 hours; in fact, some clinicians have suggested that patients should carry nifedipine with them for immediate use in the event of a hypertensive crisis.
Because of the drug interaction of the classic MAOIs with food, clinicians usually make several dietary recommendations (Table 8–3). These recommendations are quite varied.
To be avoided |
To be used in moderation |
Cheese (except cream cheese and cottage cheese) |
Coffee |
Overripe (aged) fruit (e.g., overripe bananas or avocados) |
Chocolate |
Fava beans |
Colas |
Sausage, salami |
Tea |
Beef and chicken livers |
Soy sauce |
Monosodium glutamate |
Beer, other wines |
Sauerkraut |
|
Pickled fish |
|
Brewer’s yeast |
|
Fermented products |
|
Sherry, liqueurs |
|
Red wine |
MAOI diets recommend restriction of cheese (except cream cheese and cottage cheese), red wine, sherry, liqueurs, pickled fish, overripe (aged) fruit, brewer’s yeast, fava beans, beef and chicken liver, and fermented products. Some diets recommend restriction of all alcoholic beverages, coffee, chocolate, colas, tea, yogurt, soy sauce, avocados, and bananas. Notably, many of the restricted foods—for example, avocados and bananas—rarely cause hypertensive crisis. For example, an interaction may occur only if overripe fruit is eaten or, in the case of bananas, if the skin is eaten (which is an uncommon practice in the United States). Similarly, unless a person ingests large amounts of caffeine, the interaction is usually not clinically significant. Although there is greater risk of noncompliance with highly restrictive diets, the clinician, when discussing restrictions and cautions with the patient, should emphasize the need to adhere to dietary restrictions and the potential risks that arise by breaking the diet (Gardner et al. 1996).
In evaluating patients who may have had a drug–food reaction, the clinician should evaluate the hypertensive reaction and differentiate it from histamine headache, which also can occur with an MAOI. Histamine headaches are usually accompanied by hypotension, colic, loose stools, salivation, and lacrimation (Cooper 1967). The clinician should provide both oral and printed instructions about food and drug interactions to patients who are taking classic MAOIs.
Drug–Drug Interactions
Drug–drug interactions are also extremely important concerns in patients taking irreversible MAOIs. The extensive inhibition of MAO enzymes by MAOIs raises the potential for a number of interactions with other drugs (Table 8–4). Of particular importance, many over-the-counter medications can interact with MAOIs. These medications include cough syrups containing sympathomimetic agents, which in the presence of an MAOI can precipitate a hypertensive crisis.
Drug |
Interaction |
Comment |
Other MAOIs (e.g., furazolidone, pargyline, procarbazine) |
Potentiation of side effects; convulsions possible |
Allow at least 1 week before changing to another MAOI |
Tricyclic antidepressants (TCAs) (e.g., maprotiline, bupropion) |
Possibility of severe side effects, such as hypertension and convulsions; serotonin syndrome |
Allow at least 2 weeks before changing to an MAOI; combinations have been used occasionally for refractory depression |
Carbamazepine |
Low possibility of interaction; similar to TCAs |
Same as for TCAs |
Cyclobenzaprine |
Low possibility of interaction; similar to TCAs |
Same as for TCAs |
Selective serotonin reuptake inhibitors (SSRIs) |
Serotonin syndrome |
Avoid combinations; allow at least 2 weeks before changing to an MAOI and 5 weeks if switching from fluoxetine to an MAOI |
Stimulants (e.g., methylphenidate, dextroamphetamine) |
Potential for increased blood pressure (hypertension) |
Avoid combination |
Buspirone |
Potential for increased blood pressure (hypertension) |
Avoid use; if used, monitor blood pressure |
Meperidine |
Possibility of severe, potentially fatal interaction (see text); serotonin syndrome |
Avoid combination |
Dextromethorphan |
Reports of brief psychosis |
Avoid high doses |
Direct sympathomimetics (e.g., L-dopa) |
Increased blood pressure |
Avoid use if possible; if they need to be used, use with caution |
Indirect sympathomimetics |
Hypertensive crisis possible |
Avoid use |
Oral hypoglycemics (e.g., insulin) |
Worsening of hypoglycemia possible |
Monitor blood sugar levels and adjust medications |
Fenfluramine |
Serotonin syndrome possible |
Avoid use |
L-Tryptophan |
Serotonin syndrome possible |
Avoid use |
Another area of caution is the use of MAOIs in patients who need surgery. Potential interactions include those with narcotic drugs, especially meperidine. Meperidine administered with MAOIs can produce a syndrome characterized by coma, hyperpyrexia, and hypertension. This syndrome has been reported primarily with phenelzine; however, it has also been reported with tranylcypromine (Mendelson 1979; Stack et al. 1988). Stack et al. (1988) noted that this syndrome is most likely to occur with meperidine and that it may be related to that drug’s serotonergic properties (similar to serotonin syndrome). Similar reactions have not been reported to any significant extent with other narcotic analgesics such as morphine and codeine. In fact, many patients probably receive these medications without problems. Only a small fraction of patients may have this interaction, and it could reflect a serotonin toxicity effect. In general, current opinion favors the use of morphine when intra- or postoperative narcotics are needed in patients taking MAOIs.
The issue of whether directly acting sympathomimetic amines interact with MAOIs is more controversial. Intravenous administration of sympathomimetic amines to patients receiving MAOIs does not provoke hypertension. When a bolus infusion of any of various catecholamines is given to healthy volunteer subjects who have been taking phenelzine or tranylcypromine for 1 week, a potentiation of the pressor effect of phenylephrine occurs, but no clinically significant potentiation of cardiovascular effects of NE, epinephrine, or isoproterenol occurs (Wells 1989).
In general, direct sympathomimetic amine–MAOI interactions do not appear to produce significant cardiovascular problems. However, there is a low incidence of hypertensive episodes in the presence of indirect sympathomimetics. Ideally, these compounds should not be used in patients who are receiving MAOIs. A direct-acting compound is preferable to an indirect-acting compound.
Caution should be exercised when using MAOIs in patients with pheochromocytoma or with cardiovascular, cerebrovascular, or hepatic disease. Because phenelzine tablets contain gluten, they should not be given to patients with celiac disease.
Each patient should be given a card indicating that he or she is taking an MAOI and instructed to carry the card at all times. A medical bracelet indicating that the wearer takes an MAOI is also a good idea.
Specific Monoamine Oxidase Inhibitors
Phenelzine
Phenelzine, a hydrazine derivative, is a potent irreversible MAOI and is the best studied of the MAOIs.
Pharmacokinetics
Phenelzine is a substrate as well as an inhibitor of MAO. Major identified metabolites of phenelzine include phenylacetic acid and p-hydroxyphenylacetic acid. Phenelzine undergoes acetylation, and therefore drug levels are lower in fast acetylators than in slow acetylators. However, because phenelzine is an irreversible inhibitor, plasma concentrations are not relevant. The antidepressant effect, the degree of inhibition of MAO, and the amount of free phenelzine excreted in the urine are all significantly greater in slow acetylators than in fast acetylators (Baker et al. 1999).
Indications
Phenelzine is useful in the treatment of major depressive disorder, atypical depression, panic disorder, social phobia, and atypical facial pain (see section “Indications and Efficacy” earlier in this chapter).
Side Effects
The primary side effects of phenelzine are similar to those of other MAOIs. Hepatitis secondary to phenelzine may occur in rare cases (<1 in 30,000). The most difficult side effect, often leading to discontinuation, is postural hypotension.
Contraindications
The contraindications to phenelzine include known sensitivity to the drug, pheochromocytoma, congestive heart failure, and history of liver disease. (In addition, see sections “Dietary Interactions” and “Drug–Drug Interactions” earlier in this chapter.)
Isocarboxazid
Isocarboxazid is an irreversible MAOI of the hydrazine type.
Pharmacokinetics
Isocarboxazid is rapidly absorbed from the gastrointestinal tract and is metabolized in the liver. It is primarily excreted as hippuric acid. Its half-life is of little interest because it is an irreversible MAOI. Chemically, isocarboxazid is 5-methyl-3-isoxazolecarboxylic acid 2-benzylhydrazide. Isocarboxazid is a colorless crystalline substance with very little taste.
Indications
Isocarboxazid is the least studied of the MAOIs. It is indicated for the treatment of depression.
Side Effects
The side effects of isocarboxazid are similar to those of phenelzine, described earlier in this section. Postural hypotension is the most common problem.
Contraindications
The contraindications to isocarboxazid are similar to those of phenelzine, described earlier in this section.
Tranylcypromine
Tranylcypromine, a nonhydrazine irreversible MAOI, increases the concentration of NE, epinephrine, and 5-HT in the CNS. Tranylcypromine has a mild stimulant effect.
Pharmacokinetics
Limited data exist on the pharmacokinetics of tranylcypromine. The drug is excreted within 24 hours. The dynamic effect lasts for up to 5 days after withdrawal. There is considerable debate about whether tranylcypromine is metabolized to amphetamine; most studies in the literature indicate that this does not occur.
Indications
Tranylcypromine is indicated for the treatment of major depressive disorder without melancholia.
Side Effects
The side effects of tranylcypromine are similar to those of other MAOIs. In addition, problems with physical dependence on tranylcypromine have been reported. Thus, withdrawal symptoms, such as anxiety, restlessness, depression, and headache, may occur. Syndrome of inappropriate antidiuretic hormone (SIADH) has been reported with tranylcypromine. Rare cases of toxic hepatitis have also been reported. Tranylcypromine can lead to increased agitation, insomnia, and restlessness, compared with phenelzine.
Contraindications
The contraindications to tranylcypromine are the same as those for phenelzine, described earlier in this section. In addition, in view of the greater potential for hypertensive episodes, tranylcypromine should be used with particular caution in patients with cerebrovascular or cardiovascular disease.
Moclobemide
Moclobemide, a reversible inhibitor of MAO-A enzyme (Amrein et al. 1989), has a higher potency in vivo than in vitro. Therefore, it has been suggested that moclobemide is a prodrug and that it is metabolized to a form with higher affinity for MAO-A than the parent compound. After single- or repeated-dose administration of moclobemide, the recovery of MAO-A activity is much quicker than that seen with other MAOIs. One of the metabolites of moclobemide does inhibit MAO-B; however, this action is minimally significant in humans. When administered to rats, moclobemide increases the concentration of 5-HT, NE, epinephrine, and DA in rat brain (Haefely et al. 1992). These effects are short lasting, and they parallel the time course of MAO-A inhibition. In addition, unlike with irreversible inhibitors, repeated administration does not increase the inhibition.
Moclobemide only partially potentiates the blood pressor effect of oral tyramine (DaPrada et al. 1989). This is because it is a reversible inhibitor with a low affinity for the MAO isoenzymes and is easily displaced by the pressor amines ingested in food. On the basis of these studies, moclobemide is thought to be safer than irreversible MAOIs.
Pharmacokinetics
After oral administration of moclobemide, peak plasma concentrations are reached within 1 hour. The drug is about 50% bound to plasma proteins and is extensively metabolized; only 1% of the compound is excreted (unchanged) in the urine. The half-life of the compound is approximately 12 hours. Moclobemide is extensively metabolized; 95% of the administered dose is excreted in the urine. The metabolites are pharmacologically inactive. The presence of food reduces the rate (but not the extent) of moclobemide absorption.
Indications
Moclobemide has been studied in all types of depressive disorders (Gabelic and Kuhn 1990; Larsen et al. 1991; Rossel and Moll 1990). Controlled trials have found that it is superior to placebo. In addition, moclobemide has been found to be as effective as imipramine, desipramine, clomipramine, and amitriptyline in the treatment of depression. The dosage required is 300–600 mg/day.
Unlike the classic MAOIs, moclobemide has been found to be effective in treating both endogenous and nonendogenous depression. In addition, in combination with antipsychotics, the drug seems to be effective in treating psychotic depression (Amrein et al. 1989). Moclobemide has also been effective in treating bipolar endogenous depression.
Versiani et al. (1992) compared phenelzine, moclobemide, and placebo and reported that both phenelzine and moclobemide were superior to placebo in treating patients with social phobia. Given the efficacy of classic MAOIs in the treatment of other psychiatric disorders, such as bulimia, panic disorder, and PTSD, it is likely that patients with such disorders would also respond to a reversible MAOI. Additional trials of moclobemide are required to confirm its utility in other psychiatric disorders.
Side Effects
Nausea was the only side effect noted to be greater in patients taking moclobemide than in patients taking placebo. Thus, the profile of moclobemide seems to be ideal in that it causes few or no major side effects. Case reports have shown no toxicity after overdoses of up to 20 g (Amrein et al. 1989).
Dietary Interactions
Intravenous tyramine pressor tests indicate that a single dose of moclobemide increases tyramine sensitivity (Cusson et al. 1991). However, this increase is marginal, compared with the increase associated with other MAOIs. Under most conditions, there appears to be limited drug–food interaction. However, to minimize even mild tyramine pressor effects, the recommended action is to administer moclobemide after a meal rather than before it. In a study in which tyramine was administered in doses up to 100 mg, inpatients pretreated with moclobemide had no significant changes in blood pressure. The drug also has minimal effect on cognitive performance and no effect on body weight or hematological parameters (Wesnes et al. 1989; Youdim et al. 1987).
Drug–Drug Interactions
Several studies have examined potential drug–drug interactions with moclobemide (Amrein et al. 1992). No drug interaction with lithium has been reported. No interactions with benzodiazepines or antipsychotics have been reported (Amrein et al. 1992). Parallel data suggest that moclobemide can potentiate the effects of meperidine; therefore, the narcotic–MAOI interaction may occur. Combination with other antidepressants (including SSRIs) is best avoided in view of potential serotonin toxicity. Until proven otherwise, it would be prudent to avoid the combination of moclobemide with opiates like meperidine. A pharmacokinetic interaction has been observed with cimetidine that requires the reduction of the moclobemide dose because cimetidine reduces the clearance of moclobemide.
Selegiline Hydrochloride
Selegiline hydrochloride is an irreversible MAO-B inhibitor (Cesura and Pletscher 1992). Its primary use is in the treatment of Parkinson’s disease, as an adjunct to L-dopa and carbidopa. The average dosage for Parkinson’s disease is 5–10 mg/day. The exact mechanism of action of MAO-B in Parkinson’s disease is unknown (Gerlach et al. 1996; Hagan et al. 1997; Lyytinen et al. 1997).
Pharmacokinetics
Selegiline is metabolized to levoamphetamine, methamphetamine, and N-desmethylselegiline. Selegiline hydrochloride undergoes significant first-pass metabolism following oral administration. Transdermal delivery avoids the first-pass effect and provides greater levels of unchanged drug and reduced levels of metabolites compared with the oral regimen. The time to reach the peak is less than 1 hour. The elimination half-life of selegiline is about 1.5 hours. There is at least a threefold increase in the area under the curve (AUC) of selegiline with food (Mahmood 1997).
Indications and Efficacy
The efficacy of selegiline in treating depression has not been well studied. Quitkin et al. (1984) showed that selegiline was superior to placebo administered to patients with depression in a 6-week double-blind study. Dosages of more than 10–20 mg/day were needed. The dosage required for treating depression may be much higher than that required to treat Parkinson’s disease. At higher dosages, dietary interactions could occur. Early studies also found that selegiline is of modest benefit in patients with Alzheimer’s disease (Lawlor et al. 1997).
Side Effects
The few side effects that have been noted with selegiline include nausea, dizziness, and light-headedness. When the drug is abruptly discontinued, nausea, hallucinations, and confusion have been reported.
Dietary Interactions
Because MAO-B is not involved in the intestinal tyramine interaction, dietary interaction with selegiline (at low dosages of 5–10 mg/day) would probably be minimal. An interaction between selegiline and narcotics has been reported and should be kept in mind.
Drug–Drug Interactions
Selegiline’s potential drug interactions are similar to those of other MAOIs, and there is a risk for serotonin syndrome if selegiline is combined with other drugs (including SSRIs) that can increase serotonin.
Selegiline Transdermal System
The selegiline transdermal system (STS) was developed to overcome limitations of orally administered MAOIs, particularly dietary tyramine restrictions. STS does not overcome drug–drug interactions. It bypasses the gut, thereby reducing drug–food interactions. The pharmacokinetic and pharmacodynamic properties promote the inhibition of MAO-A and MAO-B in the CNS while avoiding significant inhibition of intestinal and liver MAO-A enzymes. Three different strengths of STS patch are currently marketed: 20 mg/20 cm2, 30 mg/30 cm2, and 40 mg/40 cm2. The three patch sizes deliver 24-hour doses of selegiline averaging 6 mg, 9 mg, and 12 mg, respectively. Use of the 6-mg patch does not call for dietary modification. A restricted “MAOI diet” is advised for the higher-dosage 9-mg and 12-mg patches to avoid any risk of hypertensive crisis. Patients are strongly advised to follow these restrictions.
Pharmacokinetics
Following dermal application of the STS patch, 25%–30% of the selegiline content on average is delivered systemically over 24 hours. Consequently, the degree of drug absorption is one-third higher than the average amounts of 6–12 mg/24 hours. In comparison with oral dosing, transdermal dosing results in substantially higher exposure to selegiline and lower exposure to metabolites.
Indications and Efficacy
The efficacy of STS as a treatment for major depressive disorder was established in two placebo-controlled studies of 6 and 8 weeks’ duration in adult outpatients with major depressive disorder. In both studies, patients were randomly assigned to double-blind treatment with drug patch or placebo. The 6-week trial showed that 6 mg/24 hours was significantly more effective than placebo, as assessed by scores on the 17-item Hamilton Rating Scale for Depression (Ham-D) (Amsterdam 2003). In an 8-week dosage titration trial, depressed patients receiving the drug patch (starting dosage was 6 mg/24 hours, with possible increases to 9 mg/24 hours or 12 mg/24 hours based on clinical response) showed significant improvement compared with those receiving placebo on the primary outcome measure, the 28-item Ham-D total score (Feiger et al. 2006). In another trial, 322 patients meeting DSM-IV-TR (American Psychiatric Association 2000) criteria for major depressive disorder who had responded during an initial 10-week open-label treatment phase were randomly assigned either to continuation at the same dose or to placebo under double-blind conditions for observation of relapse. In this double-blind phase, patients receiving continued STS experienced a significantly longer time to relapse (Amsterdam and Bodkin 2006). The efficacy was also studied in a double-blind study of depressed adolescents. There was no significant difference between patients receiving STS and those receiving placebo (DelBello et al. 2014).
Side Effects
The main side effects with STS are diarrhea, skin irritation, and insomnia.
Drug–Drug Interactions
Potential drug–drug interactions for STS are the same as for other MAOIs.
Conclusion
Various MAOIs have been shown to be effective in treating a wide variety of psychiatric disorders, including depression, panic disorder, social phobia, and PTSD. The classic MAOIs are currently used only rarely as first-line medication because of potential dietary interactions and other long-term side effects. The reversible inhibitors of MAO-A enzyme, such as moclobemide, which have fewer side effects and no dietary restrictions compared with classic MAOIs, are unlikely to be introduced in the United States. In fact, the risk–benefit ratio for these compounds is highly favorable compared with other antidepressants. The MAO-B inhibitor selegiline is used to reduce the progression of Parkinson’s disease. Its utility in treating other degenerative disorders is currently being assessed. STS reduces dietary interactions when used at low doses and is now approved for the treatment of major depressive disorder. New applications and a wider use of these compounds may be found in the near future.
References
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. Arlington, VA, American Psychiatric Association, 2000
Amrein R, Allen SR, Guentert TW, et al: The pharmacology of reversible monoamine oxidase inhibitors. Br J Psychiatry Suppl (6):66–71, 1989 2695128
Amrein R, Güntert TW, Dingemanse J, et al: Interactions of moclobemide with concomitantly administered medication: evidence from pharmacological and clinical studies. Psychopharmacology (Berl) 106 (suppl):S24–S31, 1992 1546135
Amsterdam JD: A double-blind, placebo-controlled trial of the safety and efficacy of selegiline transdermal system without dietary restrictions in patients with major depressive disorder. J Clin Psychiatry 64(2):208–214, 2003 12633131
Amsterdam JD, Bodkin JA: Selegiline transdermal system in the prevention of relapse of major depressive disorder: a 52-week, double-blind, placebo-substitution, parallel-group clinical trial. J Clin Psychopharmacol 26(6):579–586, 2006 17110814
Baker GB, Urichuk LJ, McKenna KF, et al: Metabolism of monoamine oxidase inhibitors. Cell Mol Neurobiol 19(3):411–426, 1999 10319194
Blackwell B, Marley E, Price J, et al: Hypertensive interactions between monoamine oxidase inhibitors and foodstuffs. Br J Psychiatry 113(497):349–365, 1967 6034391
Bloch RG, Dooneief AS, Buchberg AS, et al: The clinical effect of isoniazid and iproniazid in the treatment of pulmonary tuberculosis. Ann Intern Med 40(5):881–900, 1954 13159064
Brunner HG, Nelen M, Breakefield XO, et al: Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262(5133): 578–580, 1993 8211186
Cesura AM, Pletscher A: The new generation of monoamine oxidase inhibitors. Prog Drug Res 38:171–297, 1992 1609114
Cheese and tranylcypromine (letter). BMJ 3(5718):354, 1970 5451971
Cipriani A, Geddes JR, Furukawa TA, et al: Metareview on short-term effectiveness and safety of antidepressants for depression: an evidence-based approach to inform clinical practice. Can J Psychiatry 52(9):553–562, 2007 17953159
Cooper AJ: M.A.O. inhibitors and headache (letter). BMJ 4(5576):420, 1967 4964185
Cooper AJ: Tyramine and irreversible monoamine oxidase inhibitors in clinical practice.. Br J Psychiatry Suppl (6):38–45, 1989 2695126
Crane GE: Iproniazid (Marsilid) phosphate, a therapeutic agent for mental disorders and debilitating diseases. Psychiatr Res Rep Am Psychiatr Assoc 8:142–152, 1957 13542682
Cronin D: Monoamine-oxidase inhibitors and cheese (letter). BMJ 2(5469):1065, 1965 5826297
Cusson JR, Goldenberg E, Larochelle P: Effect of a novel monoamine-oxidase inhibitor, moclobemide on the sensitivity to intravenous tyramine and norepinephrine in humans. J Clin Pharmacol 31(5):462–467, 1991 2050833
DaPrada M, Kettler R, Burkard W, et al: Moclobemide, an antidepressant with short-lasting MAO-A inhibition: brain catecholamines/tyramine pressor effects in rats, in Monoamine Oxidase and Disease. Edited by Tipton K, Dostert P, Strolin Benedetti M. New York, Academic Press, 1984, pp 137–154
DaPrada M, Kettler R, Keller HH, et al: Neurochemical profile of moclobemide, a short-acting and reversible inhibitor of monoamine oxidase type A. J Pharmacol Exp Ther 248(1):400–414, 1989 2783611
Davidson J, Turnbull C: Loss of appetite and weight associated with the monoamine oxidase inhibitor isocarboxazid. J Clin Psychopharmacol 2(4):263–266, 1982 7119133
Davidson J, Raft D, Pelton S: An outpatient evaluation of phenelzine and imipramine. J Clin Psychiatry 48(4):143–146, 1987a 3549705
Davidson J, Walker JI, Kilts C: A pilot study of phenelzine in the treatment of post-traumatic stress disorder. Br J Psychiatry 150:252–255, 1987b 3651684
DelBello MP, Hochadel TJ, Portland KB, et al: A double-blind, placebo-controlled study of selegiline transdermal system in depressed adolescents. J Child Adolesc Psychopharmacol 24(6):311–317, 2014 24955812
Evans DL, Davidson J, Raft D: Early and late side effects of phenelzine. J Clin Psychopharmacol 2(3):208–210, 1982 7096609
Feiger AD, Rickels K, Rynn MA, et al: Selegiline transdermal system for the treatment of major depressive disorder: an 8-week, double-blind, placebo-controlled, flexible-dose titration trial. J Clin Psychiatry 67(9):1354–1361, 2006 17017821
Finberg JPM, Youdim MBH: Reversible monoamine oxidase inhibitors and the cheese effect, in Monoamine Oxidase and Disease: Prospects for Therapy With Reversible Inhibitors. Edited by Tipton KF, Dostert P, Strolin Benedetti M. New York, Academic Press, 1984, pp 479–485
Gabelic I, Kuhn B: Moclobemide (Ro 11-1163) versus tranylcypromine in the treatment of endogenous depression (abstract). Acta Psychiatr Scand Suppl 360:63, 1990 2248076
Gardner DM, Shulman KI, Walker SE, Tailor SA: The making of a user friendly MAOI diet. J Clin Psychiatry 57(3):99–104, 1996 8617704
Gerlach M, Youdim MB, Riederer P: Pharmacology of selegiline. Neurology 47 (6 suppl 3):S137–S145, 1996 8959982
Glick R, Harrison W, Endicott J, et al: Treatment of premenstrual dysphoric symptoms in depressed women. J Am Med Womens Assoc 46(6):182–185, 1991 1744374
Goldstein DM, Goldberg RL: Monoamine oxidase inhibitor-induced speech blockage. J Clin Psychiatry 47(12):604, 1986 3782047
Goodheart RS, Dunne JW, Edis RH: Phenelzine associated peripheral neuropathy—clinical and electrophysiologic findings. Aust NZ J Med 21(3):339–340, 1991 1659356
Haefely W, Burkard WP, Cesura AM, et al: Biochemistry and pharmacology of moclobemide, a prototype RIMA. Psychopharmacology (Berl) 106 (suppl):S6–S14, 1992 1546143
Hagan JJ, Middlemiss DN, Sharpe PC, et al: Parkinson’s disease: prospects for improved drug therapy. Trends Pharmacol Sci 18(5):156–163, 1997 9184476
Harrison WM, Stewart J, Ehrhardt AA, et al: A controlled study of the effects of antidepressants on sexual function. Psychopharmacol Bull 21(1):85–88, 1985 3885294
Hedberg DL, Gordon MW, Glueck BC Jr: Six cases of hypertensive crisis in patients on tranylcypromine after eating chicken livers. Am J Psychiatry 122(8):933–937, 1966 5948152
Himmelhoch JM, Fuchs CZ, Symons BJ: A double-blind study of tranylcypromine treatment of major anergic depression. J Nerv Ment Dis 170(10):628–634, 1982 7050302
Himmelhoch JM, Thase ME, Mallinger AG, et al: Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 148(7):910–916, 1991 2053632
Husain M, Edmondson DE, Singer TP: Kinetic studies on the catalytic mechanism of liver monoamine oxidase. Biochemistry 21(3):595–600, 1982 7066309
Jacobson JN: Anorgasmia caused by an MAOI (letter). Am J Psychiatry 144(4):527, 1987 3565632
Jenike MA: Rapid response of severe obsessive-compulsive disorder to tranylcypromine. Am J Psychiatry 138(9):1249–1250, 1981 7270737
Jenike MA, Baer L, Minichiello WE, et al: Placebo-controlled trial of fluoxetine and phenelzine for obsessive-compulsive disorder. Am J Psychiatry 154(9):1261–1264, 1997 9286186
Johnstone EC: Relationship between acetylator status and response to phenelzine. Mod Probl Pharmacopsychiatry 10:30–37, 1975 1101047
Johnstone EC, Marsh W: The relationship between response to phenelzine and acetylator status in depressed patients. Proc R Soc Med 66(9):947–949, 1973 4616245
Kelly D, Mitchell-Heggs N, Sherman D: Anxiety and the effects of sodium lactate assessed clinically and physiologically. Br J Psychiatry 119(549):129–141, 1971 5565900
Kennedy SH, Piran N, Warsh JJ, et al: A trial of isocarboxazid in the treatment of bulimia nervosa. J Clin Psychopharmacol 8(6):391–396, 1988 3069879
Kline NS: Clinical experience with iproniazid (Marsilid). J Clin Exp Psychopathol 19 (2 suppl 1):72–78, discussion 78–79, 1958 13549569
Kosten TR, Frank JB, Dan E, et al: Pharmacotherapy for posttraumatic stress disorder using phenelzine or imipramine. J Nerv Ment Dis 179(6):366–370, 1991 2051152
Larsen JK, Gjerris A, Holm P, et al: Moclobemide in depression: a randomized, multicentre trial against isocarboxazide and clomipramine emphasizing atypical depression. Acta Psychiatr Scand 84(6):564–570, 1991 1792931
Lawlor BA, Aisen PS, Green C, et al: Selegiline in the treatment of behavioural disturbance in Alzheimer’s disease. Int J Geriatr Psychiatry 12(3):319–322, 1997 9152715
Liebowitz MR, Schneier F, Campeas R, et al: Phenelzine vs atenolol in social phobia. A placebo-controlled comparison. Arch Gen Psychiatry 49(4):290–300, 1992 1558463
Lima MS, Moncrieff J: Drugs versus placebo for dysthymia. Cochrane Database Syst Rev (4):CD001130, 2000 11034701
Lydiard RB, Laraia MT, Howell EF, et al: Phenelzine treatment of panic disorder: lack of effect on pyridoxal phosphate levels. J Clin Psychopharmacol 9(6):428–431, 1989 2687338
Lyytinen J, Kaakkola S, Ahtila S, et al: Simultaneous MAO-B and COMT inhibition in L-Dopa-treated patients with Parkinson’s disease. Mov Disord 12(4):497–505, 1997 9251066
Mahmood I: Clinical pharmacokinetics and pharmacodynamics of selegiline. An update. Clin Pharmacokinet 33(2):91–102, 1997 9260033
McElroy SL, Keck PE Jr, Pope HG Jr, et al: Pharmacological treatment of kleptomania and bulimia nervosa. J Clin Psychopharmacol 9(5):358–360, 1989 2677062
McGrath PJ, Stewart JW, Harrison W, et al: Phenelzine treatment of melancholia. J Clin Psychiatry 47(8):420–422, 1986 3525522
McGrath PJ, Stewart JW, Fava M, et al: Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry 163(9):1531–1541, quiz 1666, 2006 16946177
Mendelson G: Narcotics and monoamine oxidase-inhibitors (letter). Med J Aust 1(9):400, 1979 470771
Meyer JH, Wilson AA, Sagrati S, et al: Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch Gen Psychiatry 66(12):1304–1312, 2009 19996035
Moises HW, Beckmann H: Antidepressant efficacy of tranylcypromine isomers: a controlled study. J Neural Transm 50(2–4):185–192, 1981 7017068
Nolen WA: Tranylcypromine in depression resistant to cyclic antidepressions. Prog Neuropsychopharmacol Biol Psychiatry 13(1–2):155–158, 1989 2664883
Murphy DL, Garrick NA, Aulakh CS, et al: New contributions from basic science to understanding the effects of monoamine oxidase inhibiting antidepressants. J Clin Psychiatry 45(7 pt 2):37–43, 1984 6735994
Murphy DL, Sunderland T, Garrick NA, et al: Selective amine oxidase inhibitors: basic to clinical studies and back, in Clinical Pharmacology in Psychiatry. Edited by Dahl SG, Gram A, Potter W. Berlin, Springer-Verlag, 1987, pp 135–146
O’Brien S, McKeon P, O’Regan M, et al: Blood pressure effects of tranylcypromine when prescribed singly and in combination with amitriptyline. J Clin Psychopharmacol 12(2):104–109, 1992 1573032
Paykel ES, West PS, Rowan PR, et al: Influence of acetylator phenotype on antidepressant effects of phenelzine. Br J Psychiatry 141:243–248, 1982 6753997
Pearce LB, Roth JA: Human brain monoamine oxidase type B: mechanism of deamination as probed by steady-state methods. Biochemistry 24(8):1821–1826, 1985 4016087
Quitkin F, Rifkin A, Klein DF: Monoamine oxidase inhibitors. A review of antidepressant effectiveness. Arch Gen Psychiatry 36(7):749–760, 1979 454092
Quitkin FM, Liebowitz MR, Stewart JW, et al: L-Deprenyl in atypical depressives. Arch Gen Psychiatry 41(8):777–781, 1984 6430257
Quitkin FM, McGrath PJ, Stewart JW, et al: Atypical depression, panic attacks, and response to imipramine and phenelzine. A replication. Arch Gen Psychiatry 47(10):935–941, 1990 2222132
Quitkin FM, Harrison W, Stewart JW, et al: Response to phenelzine and imipramine in placebo nonresponders with atypical depression: a new application of the crossover design. Arch Gen Psychiatry 48(4):319–323, 1991 2009033
Ramsay RR, Singer TP: The kinetic mechanisms of monoamine oxidases A and B. Biochem Soc Trans 19(1):219–223, 1991 2037155
Rossel L, Moll E: Moclobemide versus tranylcypromine in the treatment of depression. Acta Psychiatr Scand Suppl 360:61–62, 1990 2248075
Rowan PR, Paykel ES, West PS, et al: Effects of phenelzine and acetylator phenotype. Neuropharmacology 20(12B):1353–1354, 1981 7322308
Saura J, Kettler R, DaPrada M, et al: Quantitative enzyme radioautography with 3H-Ro 41–1049 and 3H-Ro 19–6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci 12(5):1977–1999, 1992 1578281
Simpson GM, Gratz SS: Comparison of the pressor effect of tyramine after treatment with phenelzine and moclobemide in healthy male volunteers. Clin Pharmacol Ther 52(3):286–291, 1992 1526086
Stack CG, Rogers P, Linter SPK: Monoamine oxidase inhibitors and anaesthesia. A review. Br J Anaesth 60(2):222–227, 1988 3278728
Stein DJ, Ipser JC, Balkom AJ: Pharmacotherapy for social phobia. Cochrane Database Syst Rev (4):CD001206, 2004 15495010
Stumpf JL: Drug therapy of hypertensive crises. Clin Pharm 7(8):582–591, 1988 3048849
Thase ME, Mallinger AG, McKnight D, et al: Treatment of imipramine-resistant recurrent depression, IV: A double-blind crossover study of tranylcypromine for anergic bipolar depression. Am J Psychiatry 149(2):195–198, 1992 1734739
Tyrer P, Candy J, Kelly D: A study of the clinical effects of phenelzine and placebo in the treatment of phobic anxiety. Psychopharmacology (Berl) 32(3):237–254, 1973 4586902
Vallejo J, Gasto C, Catalan R, et al: Double-blind study of imipramine versus phenelzine in melancholias and dysthymic disorders. Br J Psychiatry 151:639–642, 1987 3446308
Versiani M, Mundim FD, Nardi AE, et al: Tranylcypromine in social phobia. J Clin Psychopharmacol 8(4):279–283, 1988 3209719
Versiani M, Nardi AE, Mundim FD, et al: Pharmacotherapy of social phobia: a controlled study with moclobemide and phenelzine. Br J Psychiatry 161:353–360, 1992 1393304
Walsh BT, Stewart JW, Roose SP, et al: A double-blind trial of phenelzine in bulimia. J Psychiatr Res 19(2–3):485–489, 1985 3900362
Walsh BT, Gladis M, Roose SP, et al: A controlled trial of phenelzine in bulimia. Psychopharmacol Bull 23(1):49–51, 1987 3299445
Wells DG: Monoamine oxidase inhibitors revisited. Can J Anaesth 36:64–74, 1989 2563341
Wesnes KA, Simpson PM, Christmas L, et al: Acute cognitive effects of moclobemide and trazodone, alone and in combination with alcohol, in the elderly. Br J Clin Pharmacol 27(5):647P–648P, 1989
White K, Razani J, Cadow B, et al: Tranylcypromine vs nortriptyline vs placebo in depressed outpatients: a controlled trial. Psychopharmacology (Berl) 82(3):258–262, 1984 6425910
Youdim MBH, DaPrada M, Amrein R (eds): The cheese effect and new reversible MAO-A inhibitors. Proceedings of the Round Table of the International Conference on New Directions in Affective Disorders, Jerusalem, Israel, April 5–9, 1987
Zajecka J, Fawcett J: Susceptibility to spontaneous MAOI hypertensive episodes (letter). J Clin Psychiatry 52(12):513–514, 1991 1752854
Zeller EA: Diamine oxidase, in The Enzymes, 2nd Edition, Vol 8. Edited by Boyer PD, Lardy H, Myrback K. London, Academic Press, 1963, pp 313–335
Zisook S: Side effects of isocarboxazid. J Clin Psychiatry 45(7 Pt 2):53–58, 1984 6376485
Zisook S, Braff DL, Click MA: Monoamine oxidase inhibitors in the treatment of atypical depression. J Clin Psychopharmacol 5(3):131–137, 1985 3889078