CHAPTER 15
Trazodone and Nefazodone
Robert N. Golden, M.D.
Karon Dawkins, M.D.
Linda Nicholas, M.D.
Trazodone was among the earliest “second generation” antidepressants to become available for clinical use in the United States in the early 1980s. Its side-effect profile and potential toxicity were considerably different from—and in many instances preferable to—those of the original antidepressants (i.e., the monoamine oxidase inhibitors [MAOIs] and tricyclic antidepressants [TCAs]). Several years later, trazodone’s pharmacological “cousin,” nefazodone, also became available.
Trazodone
History and Discovery
Trazodone was first synthesized in Italy about four decades ago, and clinical studies began in the United States in 1978. In sharp contrast to most other antidepressants available at the time, trazodone showed minimal effects on muscarinic cholinergic receptors.
In 1982, trazodone was introduced for clinical use in the United States under the brand name Desyrel. The medication is now available in generic formulation and also in an extended-release preparation (Oleptro).
Pharmacological Profile
Trazodone is a relatively weak SSRI; however, it is relatively specific for serotonin (5-HT) uptake inhibition, with minimal effects on norepinephrine (NE) or dopamine reuptake (Hyttel 1982) (Figure 15–1). Trazodone appears to increase extracellular 5-HT concentrations through a combination of mechanisms involving the 5-HT transporter (5-HTT) and the serotonin2A/2C (5-HT2A/2C) receptors (Pazzagli et al. 1999). In addition, trazodone has some 5-HT receptor antagonist activity (Haria et al. 1994). Its active metabolite, m-chlorophenylpiperazine (mCPP), is a potent direct 5-HT receptor agonist. Thus, trazodone can be viewed as a mixed serotonergic agonist–antagonist, with the relative amount of mCPP accumulation affecting the relative degree of the predominant agonist activity. Sustained administration is associated with enhanced serotonergic neurotransmission in vivo in the rat brain (Ghanbari et al. 2010).
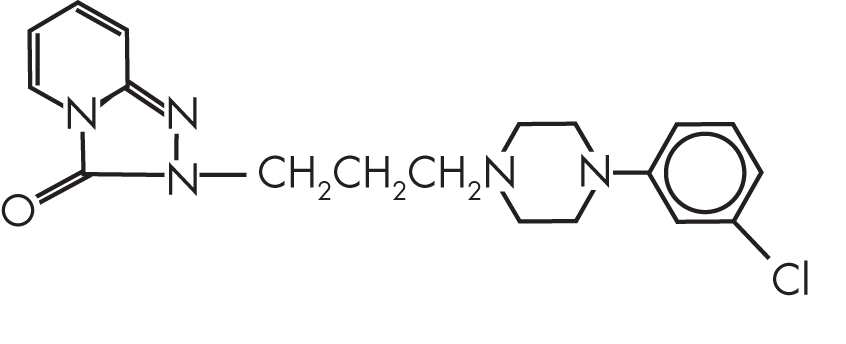
FIGURE 15–1. Chemical structure of trazodone.
In vivo, trazodone is virtually devoid of anticholinergic activity, and in clinical studies, the incidence of anticholinergic side effects is similar to that seen with placebo. Trazodone is a relatively potent antagonist of postsynaptic α1-adrenergic receptors, and it has a propensity to cause orthostatic hypotension. Trazodone has moderate antihistaminergic (histamine1 [H1] receptor) activity.
Pharmacokinetics and Disposition
Trazodone is well absorbed after oral administration, with peak blood levels occurring about 1–2 hours after dosing. Trazodone is 89%–95% bound to plasma protein. Elimination appears to be biphasic; the initial alpha and subsequent beta phases have half-lives of 3–6 and 5–9 hours, respectively. Bioavailability is not influenced by age or food intake.
Trazodone undergoes extensive hepatic metabolism. The active metabolite mCPP is cleared more slowly than the parent compound (4- to 14-hour half-life) and reaches higher concentrations in the brain than in plasma (Caccia et al. 1981). The cytochrome P450 (CYP) 2D6 and 3A microsomal enzyme systems also appear to play a role in trazodone metabolism. The relation between steady-state blood levels and clinical response to trazodone is not well defined.
Mechanism of Action
The ultimate mechanism of action of trazodone remains unclear. Although the drug is described as a 5-HT reuptake inhibitor, its effects on this neurotransmitter system are complex. Trazodone has relative selectivity for 5-HT reuptake sites (Hyttel 1982); however, in vivo, it blocks the head twitch response induced by classic 5-HT agonists in animals. The potent 5-HT receptor agonist properties of trazodone’s major metabolite, mCPP, may play a role in the mechanism of action of the parent compound. Trazodone, unlike the vast majority of antidepressants, does not produce downregulation of β-adrenergic receptors in rat cortex (Sulser 1983).
Indications and Efficacy
The primary indication for trazodone is treatment of major depressive disorder. Early reviews found that trazodone’s antidepressive efficacy was similar to that of the TCAs and the tetracyclic mianserin (Lader 1987; Schatzberg (1987).
Questions have been raised about the effectiveness of trazodone in treating severely ill depressed patients, especially those with prominent psychomotor retardation. Shopsin et al. (1981) pointed out that in several unpublished double-blind, controlled studies, the rates of clinical response to trazodone were low (i.e., 10%–20%).
The performance of trazodone, in direct comparisons with other second-generation antidepressants, has been mixed. In a double-blind, placebo-controlled comparison with venlafaxine, the final response rates were 55% for placebo, 60% for trazodone, and 72% for venlafaxine. Trazodone was more effective than venlafaxine in ameliorating sleep disturbances and was associated with the most dizziness and somnolence (Cunningham et al. 1994). In a double-blind comparison with bupropion, response rates were 46% for trazodone and 58% for bupropion (Weisler et al. 1994). In a double-blind study of 200 hospitalized patients experiencing a moderate to severe major depressive episode, mirtazapine yielded greater reductions in depression ratings than did trazodone (van Moffaert et al. 1995).
Three double-blind studies reported that trazodone had antidepressant efficacy similar to that of other antidepressants in geriatric patients (Gerner 1987). However, trazodone’s association with orthostatic hypotension may increase the risk of falls, with devastating consequences in elderly patients. Still, trazodone is often helpful for geriatric patients with depression who have severe agitation and insomnia. A survey of British geropsychiatrists identified trazodone as one of their most popular adjuncts or alternatives to atypical antipsychotics in the management of behavioral symptoms in the elderly (Condren and Cooney 2001). A Cochrane Database review found insufficient evidence to support trazodone as a treatment for the behavioral and psychological symptoms of dementia, although the review could not conclude that trazodone was ineffective, given the limited number of eligible studies (Martinon-Torres et al. 2004). A recent study of 30 patients with Alzheimer’s disease found that trazodone produced a normalization of circadian rhythms, which are often disturbed in this patient population (Grippe et al. 2015)
In a randomized, double-blind, placebo-controlled trial, the anxiolytic efficacy of trazodone was comparable to that of diazepam in weeks 3–8 of treatment for generalized anxiety disorder, although patients treated with diazepam had greater improvement during the first 2 weeks of treatment (Rickels et al. 1993).
Many clinicians use low-dose trazodone as an alternative to benzodiazepines for the treatment of insomnia. Trazodone is the second most prescribed agent for primary insomnia, even though there is minimal evidence to support its use for this indication (Mendelson 2005; Rosenberg 2006). Controlled trials have confirmed trazodone’s efficacy (at doses of 50–100 mg) in treating antidepressant-associated insomnia (Nierenberg et al. 1994). A retrospective analysis at a Department of Veterans Affairs (VA) medical center found that approximately 24% of patients receiving trazodone were taking other primary antidepressants (Clark and Alexander 2000). Another VA study of patients with posttraumatic stress disorder (PTSD) found that of those patients who were able to tolerate trazodone (60 of 72 patients), 92% reported that it improved sleep onset and 78% reported that it improved sleep maintenance (Warner et al. 2001). A recent study found that trazodone improved the Apnea-Hypopnea Index in patients with obstructive sleep apnea (OSA) without any deleterious effects on oxygen saturation or non–rapid eye movement (REM) arousal threshold, suggesting that the drug might have potential as a treatment for OSA (Smales et al. 2015).
Trazodone is more effective than placebo when added to antipsychotic medication in the treatment of the negative symptoms of schizophrenia (Singh et al. 2010; Watanabe 2011). A double-blind, placebo-controlled trial found trazodone to be effective in treating antipsychotic-induced akathisia (Stryjer et al. 2010).
A recent review highlighted the common off-label use of trazodone in a number of conditions, including bulimia, fibromyalgia, chronic pain, and diabetic neuropathy (Bossini et al. 2015). We agree with the authors’ conclusion that large randomized controlled trials are needed to determine whether there is adequate scientific evidence to support trazodone’s use for any of these indications.
Trazodone should be initiated at a low dose and increased gradually, based on clinical response and tolerance to side effects. For the treatment of a major depressive episode, the suggested initial dosage is 150 mg/day, with increases of 50-mg increments every 3–4 days. Doses may be divided, although many patients prefer bedtime dosing because of the sedating effects. The maximum dosage recommended for outpatients is 400 mg/day, although for inpatients with more severe depression, dosages up to 600 mg/day have been used. When trazodone is prescribed as a hypnotic agent, the usual dose is 50 mg at bedtime, although some patients may require as little as 25 mg or as much as 200–300 mg.
Side Effects and Toxicology
Because of its lack of anticholinergic side effects, trazodone is especially useful for patients with prostatic hypertrophy, closed-angle glaucoma, or severe constipation. Trazodone’s propensity to cause sedation is a dual-edged sword. For many patients, the relief from agitation, anxiety, and insomnia can be rapid; for others, including those with psychomotor retardation and low energy, trazodone may not be tolerable.
Trazodone was found to be among the top three medications associated with orthostatic hypotension in patients attending a VA geriatric clinic (Poon and Braun 2005). More than 200 cases of trazodone-associated priapism have been reported (Thompson et al. 1990), and the manufacturer estimates that the incidence of any abnormal erectile function is approximately 1 in 6,000 male patients. The risk appears to be greatest during the first month of treatment at low dosages (<150 mg/day). Early recognition of any abnormal erectile function is important and should prompt immediate discontinuation of trazodone treatment.
In overdose situations, trazodone appears to be relatively safer than TCAs, MAOIs, and a few of the other second-generation antidepressants, especially when it is the only agent taken. Fatalities are rare, and uneventful recoveries have been reported after ingestion of doses as high as 6,000–9,200 mg (Ayd 1984). When trazodone overdoses occur, clinicians should carefully monitor for hypotension.
In common with several other sedative-hypnotics, trazodone has the potential to impair driving skills, especially in new users, who have been shown to have an increased risk of motor vehicle crashes. The risk estimate for trazodone is roughly comparable to that of blood alcohol concentration levels of 0.09% (Hansen et al. 2015).
Drug–Drug Interactions
Trazodone can potentiate the effects of other central nervous system (CNS) depressants. Patients should be warned about increased drowsiness and sedation when trazodone is combined with other CNS depressants, including alcohol.
The combination of trazodone with an MAOI, as with other antidepressants, should be handled with great caution, although there are case reports of the successful combination of trazodone with an MAOI. Development of the serotonin syndrome has been associated with the combination of trazodone with other proserotonergic agents. Trazodone inhibits the antihypertensive effects of clonidine. Trazodone can cause hypotension, especially orthostatic hypotension, and concomitant administration of trazodone with antihypertensive therapy may require a reduction in the dose of the antihypertensive agent.
Clinically significant cases of suspected trazodone–warfarin interactions have been described.
Nefazodone
History and Discovery
Trazodone’s sedative properties and association with orthostatic hypotension inspired an effort to discover a modified molecule that would possess a more desirable pharmacological profile. This led to the development of nefazodone (Figure 15–2), which became available in the United States in 1994. Nefazodone and trazodone share a common active metabolite.
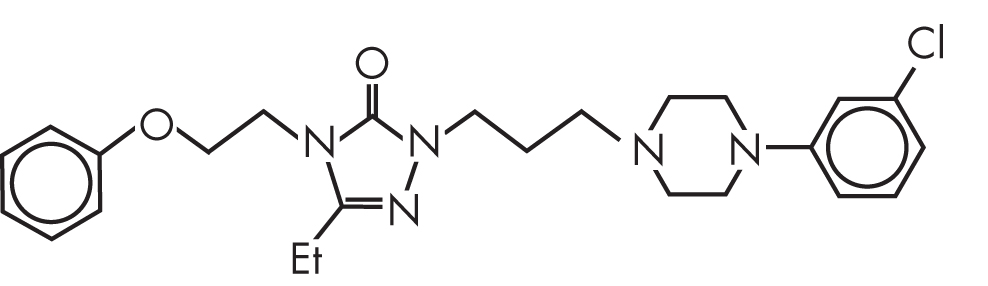
FIGURE 15–2. Chemical structure of nefazodone.
In 2004, the manufacturer of Serzone (nefazodone) announced that it was discontinuing the drug’s sale in the United States, citing declining sales. The drug had been banned in many countries because of its association with liver toxicity, and lawsuits against that manufacturer and the FDA had been initiated in this country. Nefazodone continues to be available in the United States as a generic medication.
Pharmacological Profile
Nefazodone is a 5-HT2 receptor antagonist and a weak inhibitor of 5-HT and NE reuptake (Figure 15–2). It has little affinity for α2-adrenergic, β-adrenergic, or serotonin1A (5-HT1A) receptors, and its affinity for the α1-adrenergic receptor is less than that of trazodone. Nefazodone is inactive at most other receptor-binding sites (Taylor et al. 1986).
Nefazodone demonstrates several of the classic preclinical characteristics of antidepressants. In humans, nefazodone does not suppress REM sleep, in contrast to most other antidepressants (Sharpley et al. 1996).
Pharmacokinetics and Disposition
Nefazodone is rapidly and completely absorbed and is then extensively metabolized, resulting in a low (about 20%) and variable absolute bioavailability. The plasma half-life is only 2–4 hours. Nefazodone has three active metabolites: triazole dione, hydroxynefazodone, and mCPP. Triazole dione is a specific 5-HT2 receptor antagonist with weaker affinity for that receptor than the parent compound and no appreciable effects on 5-HT reuptake. With a plasma half-life of 18 hours, triazole dione predominates in the plasma, occurring at concentrations approaching four times that of the parent compound. Hydroxynefazodone has affinities for the 5-HT2 receptor and 5-HT reuptake site that are similar to those of the parent compound. Its plasma half-life is between 1.5 and 4 hours, and at steady state, plasma concentrations are approximately 40% of those of the parent compound. mCPP is a direct agonist at the 5-HT1, 5-HT2, and serotonin3 (5-HT3) receptors, with one order of magnitude higher affinity for 5-HT2C receptors. mCPP has a plasma half-life of 4–8 hours, and its plasma concentrations are only 7% of those seen with the parent compound (DeVane et al. 2002). However, the ratios of mCPP to nefazodone concentrations in the brain are 47:1 and 10:1 in the mouse and rat, respectively. Brain concentrations of hydroxynefazodone in the rat are less than 10% of those in plasma, suggesting very poor blood–brain barrier penetration. Thus, despite its relatively lower plasma concentrations, mCPP has substantial presence in the brain, whereas the in vivo activity of hydroxynefazodone may be mostly the result of its biotransformation to mCPP (Nacca et al. 1998).
Nefazodone has nonlinear kinetics, which results in greater than proportional mean plasma concentrations with higher doses. Nefazodone is extensively (99%) but loosely protein bound (Bristol-Myers Squibb 2003). In patients with hepatic cirrhosis, single-dose nefazodone and hydroxynefazodone levels are about twice as high as in healthy volunteers, but the difference decreases to approximately 25% at steady state. Exposure to mCPP is about two- to threefold greater in patients with cirrhosis, and exposure to triazole dione is similar after a single dose and at steady state (Barbhaiya et al. 1995).
Mechanism of Action
The mechanism of action of nefazodone is poorly understood. The manufacturer has indicated that nefazodone antagonizes 5-HT2 receptors and also inhibits neuronal uptake of both 5-HT and NE (Bristol-Myers Squibb 2003). Several reviews refer to nefazodone as a “dual acting” antidepressant, suggesting that it enhances both serotonergic and noradrenergic neurotransmission via uptake blockade. Although nefazodone has similar effects on the 5-HT and NE transporters, this observation is potentially misleading. Nefazodone’s inhibition of NE reuptake is weaker than that of the SSRI fluoxetine and is approximately three orders of magnitude weaker than what is seen with conventional NE reuptake inhibitors. Furthermore, nefazodone’s inhibition of 5-HT reuptake is nearly identical to that of desipramine and more than 100-fold less than that of fluoxetine (Bolden-Watson and Richelson 1993). Thus, the “dual action” of nefazodone refers to minimal, albeit equal, effects on 5-HT and NE reuptake inhibition.
In humans, therapeutic doses of nefazodone do not cause sustained 5-HT uptake inhibition at the platelet 5-HTT (Narayan et al. 1998). The active metabolite m-CCP, which appears to predominate in the brain because of greater penetration of the blood–brain barrier (Nacca et al. 1998), may play an important role in the mechanism of action.
Indications and Efficacy
In three of four Phase III imipramine- and placebo-controlled studies, nefazodone was found to be an effective antidepressant with similar efficacy to imipramine; in one of these studies, neither active drug had significantly greater efficacy than did placebo. The incidence of premature treatment discontinuation and side effects was higher for the imipramine group than for the nefazodone treatment group (Rickels et al. 1995). In double-blind studies without placebo control groups, there were no significant differences in the clinical responses to nefazodone and sertraline or paroxetine in outpatients with depression (Feiger et al. 1996). Hospitalized patients with severe major depressive disorder had higher response rates to nefazodone compared with placebo (Feighner et al. 1998). In patients with moderate to severe major depression, the efficacy of amitriptyline was clearly superior to that of nefazodone (Ansseau et al. 1994). Keller et al. (2000) compared nefazodone, cognitive-behavioral therapy (CBT), and a combination of these two treatments in a double-blind study of patients with chronic major depressive disorder. Each monotherapy yielded a response rate of 48%, whereas the combined treatment had a greater efficacy (73%). When patients who failed to respond to 12 weeks of treatment with either nefazodone or cognitive-behavioral analysis system psychotherapy are then switched to the other treatment, significant symptom improvement is achieved (Schatzberg et al. 2005). Nefazodone has also been shown to be effective in the continuation phase of treatment in double-blind studies (Baldwin et al. 2001; Feiger et al. 1999).
In a double-blind, placebo-controlled study, nefazodone was found to be safe and effective in the treatment of depression in patients with alcohol dependence, although it did not add any advantage over psychoeducational group intervention in terms of drinking outcomes (Roy-Byrne et al. 2000). A double-blind, controlled study found that nefazodone was not efficacious for the treatment of alcohol dependence (Kranzler et al. 2000). A randomized, placebo-controlled, double-blind multicenter study compared nefazodone versus placebo and CBT versus nondirective group counseling (GC) for relapse prevention in alcohol dependence. Two hundred forty-two male patients received either nefazodone plus GC or CBT or placebo plus GC or CBT. There were no differences among the four groups in cumulative days of abstinence or amount of alcohol consumed during specified time periods during the initial 12-week study phase. After 1 year, the only significant difference among the groups was higher alcohol consumption in the nefazodone plus GC group, raising concerns that nefazodone may potentially increase the risk of relapse (Wetzel et al. 2004). Other potential clinical applications for nefazodone have been explored, including treatment of PTSSD (for which it is considered a second-line agent) (Jeffreys et al. 2012); however, nefazodone’s current use is relatively limited.
The usual starting dosage of nefazodone is 200 mg/day in two divided doses. The suggested dosage range is 300–600 mg/day. Increases should be in increments of 100–200 mg/day at weekly intervals. The starting dosage in elderly or debilitated patients should be lowered to 100 mg/day, taken in two divided doses, and the rate of titration should be adjusted accordingly (Bristol-Myers Squibb 2003). Zajecka et al. (2002) reported that in studies comparing low-dosage (50–250 mg/day) and high-dosage (100–500 mg/day) nefazodone, better clinical response was obtained in the latter group, and the mean effective dosage ranged from 375 mg/day to 460 mg/day. A lower starting dose should be considered when switching to nefazodone from an SSRI if a full washout has not been completed. Once-daily bedtime dosing appears to be well tolerated and effective.
Side Effects and Toxicology
In initial clinical trials that included approximately 2,250 patients, side effects more frequently associated with nefazodone than with placebo included dizziness, asthenia, dry mouth, nausea, and constipation (Fontaine 1993).
Preskorn (1995) found that the total cumulative incidence of treatment-emergent adverse effects for nefazodone was lower than that of imipramine or fluoxetine. The most common placebo-adjusted adverse effects associated with nefazodone were dry mouth, somnolence, dizziness, nausea, constipation, blurred vision, and postural hypotension. Nefazodone appears to have advantages over SSRIs in terms of treatment-associated sexual dysfunction (Clayton et al. 2014; Ferguson et al. 2001).
There are now well-publicized concerns regarding the association of nefazodone with liver toxicity and liver failure, including fatalities (Choi 2003; Voican et al. 2014). In 2001 the manufacturer added a black box warning to the package insert, describing a reported rate of life-threatening liver failure in the United States of 1 case per 250,000–300,000 patient-years of nefazodone treatment. In 2004 Serzone was withdrawn from the U.S. market, following its withdrawal from several international markets. The generic drug remains available in the United States. In a review of 1,338 humans with exposure to nefazodone overdoses, there were no reported deaths. The most common serious clinical effect was hypotension, reported in 1.6% of cases (Benson et al. 2000).
Drug–Drug Interactions
The manufacturer of triazolam warns that its concurrent use with nefazodone is contraindicated. Increases in the plasma concentration of digoxin occur with concurrent nefazodone administration. Nefazodone increases the plasma concentrations of terfenadine and loratadine (with associated QTc prolongation), carbamazepine, and cyclosporine.
Conclusion
Trazodone was one of the earliest second-generation antidepressants. Its lack of anticholinergic effects provided an advantage over the TCAs for many patients; its sedative properties are helpful for some patients but problematic for others; and orthostatic hypotension is a concern for elderly patients. Nefazodone is related to trazodone, and the two drugs share an active metabolite, mCPP, that may play an important role in their mechanism of action. The risk of serious liver damage led to Serzone’s removal from the market in several countries, although generic nefazodone is currently available in the United States.
References
Ansseau M, Darimont P, Lecoq A, et al: Controlled comparison of nefazodone and amitriptyline in major depressive inpatients. Psychopharmacology (Berl) 115(1–2):254–260, 1994 7862904
Ayd FJ Jr: Pharmacology update: which antidepressant to choose, II: the overdose factor. Psychiatr Ann 14(3):212–214, 1984
Baldwin DS, Hawley CJ, Mellors K; CN104-070 Study Group: A randomized, double-blind controlled comparison of nefazodone and paroxetine in the treatment of depression: safety, tolerability and efficacy in continuation phase treatment. J Psychopharmacol 15(3):161–165, 2001 11565622
Barbhaiya RH, Shukla UA, Natarajan CS, et al: Single- and multiple-dose pharmacokinetics of nefazodone in patients with hepatic cirrhosis. Clin Pharmacol Ther 58(4):390–398, 1995 7586930
Benson BE, Mathiason M, Dahl B, et al: Toxicities and outcomes associated with nefazodone poisoning: an analysis of 1,338 exposures. Am J Emerg Med 18(5):587–592, 2000 10999575
Bolden-Watson C, Richelson E: Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci 52(12):1023–1029, 1993 8445992
Bossini L, Coluccia A, Casolaro I, et al: Off-label trazodone prescription: evidence, benefits and risks. Curr Pharm Des 21(23): 3343–3351, 2015 26088119
Bristol-Myers Squibb: Serzone (nefazodone hydrochloride) tablets [packet insert]. Revised September 2003. Available at: http://www.fda.gov/ohrms/dockets/ac/04/briefing/4006B1_11_Serzone-Label.pdf. Accessed July 2012.
Caccia S, Ballabio M, Fanelli R, et al: Determination of plasma and brain concentrations of trazodone and its metabolite, 1-m-chlorophenylpiperazine, by gas-liquid chromatography. J Chromatogr A 210(2): 311–318, 1981 7263792
Choi S: Nefazodone (Serzone) withdrawn because of hepatotoxicity. CMAJ 169(11): 1187, 2003 14638657
Clark NA, Alexander B: Increased rate of trazodone prescribing with bupropion and selective serotonin-reuptake inhibitors versus tricyclic antidepressants. Ann Pharmacother 34(9):1007–1012, 2000 10981245
Clayton AH, Croft HA, Handiwala L: Antidepressants and sexual dysfunction: mechanisms and clinical implications. Postgrad Med 126(2):91–99, 2014 24685972
Condren RM, Cooney C: Use of drugs by Old Age Psychiatrists in the treatment of psychotic and behavioural symptoms in patients with dementia. Aging Ment Health 5(3):235–241, 2001 11575062
Cunningham LA, Borison RL, Carman JS, et al: A comparison of venlafaxine, trazodone, and placebo in major depression. J Clin Psychopharmacol 14(2):99–106, 1994 8195464
DeVane CL, Grothe DR, Smith SL: Pharmacology of antidepressants: focus on nefazodone. J Clin Psychiatry 63 (suppl 1):10–17, 2002 11890560
Feiger A, Kiev A, Shrivastava RK, et al: Nefazodone versus sertraline in outpatients with major depression: focus on efficacy, tolerability, and effects on sexual function and satisfaction. J Clin Psychiatry 57 (suppl 2):53–62, 1996 8626364
Feiger AD, Bielski RJ, Bremner J, et al: Double-blind, placebo-substitution study of nefazodone in the prevention of relapse during continuation treatment of outpatients with major depression. Int Clin Psychopharmacol 14(1):19–28, 1999 10221638
Feighner J, Targum SD, Bennett ME, et al: A double-blind, placebo-controlled trial of nefazodone in the treatment of patients hospitalized for major depression. J Clin Psychiatry 59(5):246–253, 1998 9632036
Ferguson JM, Shrivastava RK, Stahl SM, et al: Reemergence of sexual dysfunction in patients with major depressive disorder: double-blind comparison of nefazodone and sertraline. J Clin Psychiatry 62(1):24–29, 2001 11235924
Fontaine R: Novel serotonergic mechanisms and clinical experience with nefazodone. Clin Neuropharmacol 16 (suppl 3):S45–S50, 1993 8131154
Gerner RH: Geriatric depression and treatment with trazodone. Psychopathology 20 (suppl 1):82–91, 1987 3321134
Ghanbari R, El Mansari M, Blier P: Sustained administration of trazodone enhances serotonergic neurotransmission: in vivo electrophysiological study in the rat brain. J Pharmacol Exp Ther 335(1):197–206, 2010 20647493
Grippe TC, Gonçalves BS, Louzada LL, et al: Circadian rhythm in Alzheimer disease after trazodone use. Chronobiol Int 32(9):1311–1314, 2015 26376345
Hansen RN, Boudreau DM, Ebel BE, et al: Sedative hypnotic medication use and the risk of motor vehicle crash. Am J Public Health 105(8):e64–e69, 2015 26066943
Haria M, Fitton A, McTavish D: Trazodone. A review of its pharmacology, therapeutic use in depression and therapeutic potential in other disorders. Drugs Aging 4(4):331–355, 1994 8019056
Hyttel J: Citalopram-pharmacological profile of a specific serotonin uptake inhibitor with antidepressant activity. Prog Neuropsychopharmacol Biol Psychiatry 6(3):277–295, 1982 6128769
Jeffreys M, Capehart B, Friedman MJ: Pharmacotherapy for posttraumatic stress disorder: review with clinical applications. J Rehabil Res Dev 49(5):703–715, 2012 23015581
Keller MB, McCullough JP, Klein DN, et al: A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 342(20):1462–1470, 2000 10816183
Kranzler HR, Modesto-Lowe V, Van Kirk J: Naltrexone vs. nefazodone for treatment of alcohol dependence. A placebo-controlled trial. Neuropsychopharmacology 22(5):493–503, 2000 10731624
Lader M: Recent experience with trazodone. Psychopathology 20 (suppl 1):39–47, 1987 3321129
Martinon-Torres G, Fioravanti M, Grimley EJ: Trazodone for agitation in dementia. Cochrane Database Syst Rev (4):CD004990, 2004 15495135
Mendelson WB: A review of the evidence for the efficacy and safety of trazodone in insomnia. J Clin Psychiatry 66(4):469–476, 2005 15816789
Nacca A, Guiso G, Fracasso C, et al: Brain-to-blood partition and in vivo inhibition of 5-hydroxytryptamine reuptake and quipazine-mediated behaviour of nefazodone and its main active metabolites in rodents. Br J Pharmacol 125(7):1617–1623, 1998 9884092
Narayan M, Anderson G, Cellar J, et al: Serotonin transporter-blocking properties of nefazodone assessed by measurement of platelet serotonin. J Clin Psychopharmacol 18(1):67–71, 1998 9472845
Nierenberg AA, Adler LA, Peselow E, et al: Trazodone for antidepressant-associated insomnia. Am J Psychiatry 151(7):1069–1072, 1994 8010365
Pazzagli M, Giovannini MG, Pepeu G: Trazodone increases extracellular serotonin levels in the frontal cortex of rats. Eur J Pharmacol 383(3):249–257, 1999 10594316
Poon IO, Braun U: High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther 30(2):173–178, 2005 15811171
Preskorn SH: Comparison of the tolerability of bupropion, fluoxetine, imipramine, nefazodone, paroxetine, sertraline, and venlafaxine. J Clin Psychiatry 56 (suppl 6):12–21, 1995 7649968
Rickels K, Downing R, Schweizer E, Hassman H: Antidepressants for the treatment of generalized anxiety disorder. A placebo-controlled comparison of imipramine, trazodone, and diazepam. Arch Gen Psychiatry 50(11):884–895, 1993 8215814
Rickels K, Robinson DS, Schweizer E, et al: Nefazodone: aspects of efficacy. J Clin Psychiatry 56 (suppl 6):43–46, 1995 7649973
Rosenberg RP: Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies. Ann Clin Psychiatry 18(1):49–56, 2006 16517453
Roy-Byrne PP, Pages KP, Russo JE, et al: Nefazodone treatment of major depression in alcohol-dependent patients: a double-blind, placebo-controlled trial. J Clin Psychopharmacol 20(2):129–136, 2000 10770449
Schatzberg AF: Trazodone: a 5-year review of antidepressant efficacy. Psychopathology 20 (suppl 1):48–56, 1987 3321130
Schatzberg AF, Rush AJ, Arnow BA, et al: Chronic depression: medication (nefazodone) or psychotherapy (CBASP) is effective when the other is not. Arch Gen Psychiatry 62(5):513–520, 2005 15867104
Sharpley AL, Williamson DJ, Attenburrow ME, et al: The effects of paroxetine and nefazodone on sleep: a placebo controlled trial. Psychopharmacology (Berl) 126(1):50–54, 1996 8853216
Shopsin B, Cassano GB, Conti L: An overview of new “second generation” antidepressant compounds: research and treatment implications, in Antidepressants: Neurochemical, Behavioral and Clinical Perspectives. Edited by Enna SJ, Molick J, Richelson E. New York, Raven, 1981, pp 219–251
Singh SP, Singh V, Kar N, Chan K: Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis. Br J Psychiatry 197(3):174–179, 2010 20807960
Smales ET, Edwards BA, Deyoung PN, et al: Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc 12(5):758–764, 2015 25719754
Stryjer R, Rosenzcwaig S, Bar F, et al: Trazodone for the treatment of neuroleptic-induced acute akathisia: a placebo-controlled, double-blind, crossover study. Clin Neuropharmacol 33(5):219–222, 2010 20838215
Sulser F: Mode of action of antidepressant drugs. J Clin Psychiatry 44(5 Pt 2):14–20, 1983 6406444
Taylor DP, Smith DW, Hyslop DK, et al: Receptor binding and atypical antidepressant drug discovery, in Receptor Binding in Drug Research. Edited by O’Brien RA. New York, Marcel Dekker, 1986, pp 151–165
Thompson JW Jr, Ware MR, Blashfield RK: Psychotropic medication and priapism: a comprehensive review. J Clin Psychiatry 51(10):430–433, 1990 2211542
van Moffaert M, de Wilde J, Vereecken A, et al: Mirtazapine is more effective than trazodone: a double-blind controlled study in hospitalized patients with major depression. Int Clin Psychopharmacol 10(1):3–9, 1995 7622801
Voican CS, Corruble E, Naveau S, Perlemuter G: Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry 171(4):404–415, 2014 24362450
Warner MD, Dorn MR, Peabody CA: Survey on the usefulness of trazodone in patients with PTSD with insomnia or nightmares. Pharmacopsychiatry 34(4):128–131, 2001 11518472
Watanabe N: Fluoxetine, trazodone and ritanserin are more effective than placebo when used as add-on therapies for negative symptoms of schizophrenia. Evid Based Ment Health 14(1):21, 2011 21266618
Weisler RH, Johnston JA, Lineberry CG, et al: Comparison of bupropion and trazodone for the treatment of major depression. J Clin Psychopharmacol 14(3):170–179, 1994 8027413
Wetzel H, Szegedi A, Scheurich A, et al; NeVeR Study Group: Combination treatment with nefazodone and cognitive-behavioral therapy for relapse prevention in alcohol-dependent men: a randomized controlled study. J Clin Psychiatry 65(10):1406–1413, 2004 15491246
Zajecka J, McEnany GW, Lusk KM: Antidepressant dosing and switching guidelines: focus on nefazodone. J Clin Psychiatry 63 (suppl 1):42–47, 2002 11890564