CHAPTER 16
Vortioxetine
Pierre Blier, M.D., Ph.D.
History and Discovery
Vortioxetine, formerly designated Lu AA 21004, was developed by Lundbeck in an attempt to add to a selective serotonin (5-hydroxytryptamine [5-HT]) reuptake inhibitor (SSRI) additional properties, namely, 5-HT1A and 5-HT3 receptor affinities (Bang-Andersen et al. 2011). These pharmacological targets were based on the premises that the antidepressant effect of SSRIs can be enhanced by combining them with a partial 5-HT1A agonist and that 5-HT3 antagonists are antiemetic drugs, nausea being a common side effect of SSRIs (Sanchez et al. 2015). In 2013, vortioxetine, marketed under the brand name Brintellix, received approval from the U.S. Food and Drug Administration (FDA) and the European Medicines Agency for the treatment of major depressive disorder (Takeda Pharmaceuticals America 2016). In the United States, at the behest of the FDA, the brand name was subsequently changed to Trintellix to avoid confusion with the anticoagulant drug Brilinta (ticagrelor).
Structure–Activity Relations
Vortioxetine is a piperazine derivative, and its chemical name is 1-[2-(2,4-dimethylphenyl-sulfanyl)-phenyl]-piperazine (Figure 16–1).
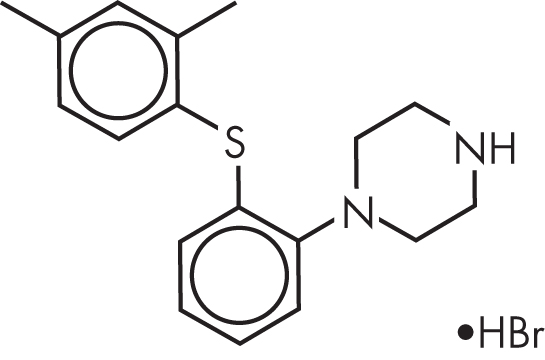
FIGURE 16–1. Chemical structure of vortioxetine.
The medication is produced in the form of a hydrobromide salt.
Pharmacological Profile
Vortioxetine is described as a multimodal serotonin agent because it acts on two types of neuronal elements: the 5-HT transporter and several 5-HT receptor subtypes (Zohar et al. 2015). Among monoamine transporters, it binds selectively to the 5-HT transporter, but only to the same extent as other SSRIs at its maximum recommended dosage, as shown in positron emission tomography studies in humans (Areberg et al. 2012; Meyer et al. 2004; Stenkrona et al. 2013). Vortioxetine is a 5-HT3 antagonist, a full 5-HT1A agonist, a 5-HT7 antagonist, a partial 5-HT1B agonist, and a 5-HT1D antagonist (Mørk et al. 2012). On the basis of plasma levels and affinity values, all of these targets could be engaged by vortioxetine to physiologically relevant levels within its usual therapeutic dosage range. Although the clinical significance of these various activities at 5-HT receptors in the presence of lower occupancy of 5-HT transporters has not been determined, synergies between such neuronal elements on neurotransmitter levels have been documented in the brains of laboratory animals (Sanchez et al. 2015).
Pharmacokinetics and Disposition
Vortioxetine is well absorbed from the gastrointestinal tract, and its bioavailability is similar under fasting and fed conditions. It reaches peak plasma concentrations in 7–11 hours and is 98% bound to plasma proteins. Vortioxetine is not a substrate for the permeability glycoproteins, indicating that brain levels will not be affected by possible polymorphisms of these efflux carriers. The terminal half-life of vortioxetine is about 66 hours, and steady-state concentrations are achieved after 2 weeks; consequently, complete elimination requires 2 weeks as well (Areberg et al. 2014; Bundgaard et al. 2016).
Vortioxetine is extensively metabolized through oxidation and subsequently by glucuronic conjugation. The cytochrome P450 (CYP) 2D6 isoenzyme is the main enzyme catalyzing its catabolism to its major carboxylic acid metabolite, which is pharmacologically inactive. About two-thirds of the inactive metabolites are excreted in the urine, and the last third are excreted in the feces. A very small amount of unchanged vortioxetine is excreted in the urine. No dosage adjustment is necessary in patients with renal impairment or mild to moderate hepatic impairment; however, vortioxetine is not recommended in patients with severe hepatic insufficiency. The plasma levels of vortioxetine are about two times higher in poor metabolizers of CYP2D6 (Chen et al. 2013).
Indications and Efficacy
At present, major depressive disorder is the only approved indication for vortioxetine worldwide. However, vortioxetine’s efficacy has also been examined in generalized anxiety disorder. Of the five short-term placebo-controlled studies and one large relapse prevention trial conducted (Fu et al. 2016), one short-term trial (Bidzan et al. 2012) and the relapse prevention trial (Baldwin et al. 2012b) reported positive findings. Although methodological issues may have been at play in the failed trials, there is not yet enough evidence to proceed with a vortioxetine indication for generalized anxiety disorder.
In contrast, the clinical development program for vortioxetine in major depressive disorder has been successful: thus far, 8 of the 12 placebo-controlled short-term studies, including one in elderly patients, have reported positive results, and a 24-week double-blind relapse prevention study also reported positive findings (Boulenger et al. 2012; Katona et al. 2012; Mahableshwarkar et al. 2015; McIntyre et al. 2014; Sanchez et al. 2015). Five of these studies included an active comparator—venlafaxine in one study, and duloxetine in the other four studies. Among patients participating in the acute studies, 2,080 received a placebo, 140 received 1 mg/day, 308 received 2.5 mg/day, 1,014 received 5 mg/day, 969 received 10 mg/day, 599 received 15 mg/day, and 615 received 20 mg/day (Table 16–1). On the basis of such results, the recommended effective dosage range is 5–20 mg/day. In adults ages 18–65 years, the usual recommended starting dosage is 10 mg/day, usually taken in the morning after a meal for convenience, but it can also be taken at night. In elderly patients, the starting dosage should be 5 mg/day. Although in the controlled studies the dosage was commonly increased after 1 week, in clinical practice a minimum of 2 weeks should elapse before an uptitration is implemented, mainly because this is the interval needed to achieve a steady-state concentration. One open-label study has assessed the safety, tolerability, and maintained effectiveness of vortioxetine in 535 patients with major depressive disorder over 52 weeks (Baldwin et al. 2016).
Study |
Duration (weeks) |
Sample size (n) |
Vortioxetine dosage (mg/day, n) |
Comparator used (n) |
Comparator dosage (mg/day) |
Study resultsa |
Findings |
6 |
429 |
5 (108) 10 (100) |
Venlafaxine (112) |
225 |
Positive |
All groups>placebo |
|
8 |
560 |
1 (140) 5 (140) 10 (140) |
None |
— |
Positive |
All groups>placebo |
|
8 |
453 |
5 (157) |
Duloxetine (151) |
60 |
Positive |
Both groups separated from placebo |
|
8 |
462 |
10 (155) 20 (150) |
None |
— |
Positive |
Vortioxetine 10 mg/day did not separate fromplacebo |
|
8 |
608 |
15 (149) 20 (151) |
Duloxetine (146) |
60 |
Positive |
All groups>placebo |
|
8 |
602 |
10 (195) 20 (207) |
None |
— |
Positive |
Both groups>placebo |
|
8 |
549 |
10–20 (175) |
Duloxetine (187) |
60 |
Positive |
Both groups>placebo |
|
8 |
614 |
15 (147) 20 (152) |
Duloxetine (152) |
60 |
Positive |
Vortioxetine 15 mg/day did not separate fromplacebo |
|
8 |
776 |
2.5 (155) 5 (157) 10 (151) |
Duloxetine (155) |
60 |
Failed |
None of the groups separated from placebo |
|
6 |
597 |
5 (299) |
None |
— |
Failed |
Vortioxetine did not separate fromplacebo |
|
8 |
469 |
10 (157) 15 (152) |
None |
— |
Failed |
Vortioxetine did not separate fromplacebo |
|
8 |
611 |
2.5 (153) 5 (153) |
Duloxetine (152) |
60 |
Negative |
Duloxetine separated fromplacebo, but vortioxetine did not |
|
Note. “>” denotes significantly greater effect. aA Positive study is one in which at least one vortioxetine arm statistically separated from placebo on the primary efficacy measure, a Failed study is a trial in which treatment group(s) did not separate from placebo, and a Negative study is one in which the comparator separated from placebo but vortioxetine did not. |
|||||||
One double-blind study examined the efficacy and tolerability of vortioxetine in 493 patients with major depressive disorder who had not responded adequately to an SSRI or a serotonin–norepinephrine reuptake inhibitor (SNRI) (Montgomery et al. 2014). In this study, patients were asked to decrease their current drug regimen to the minimum effective dosage in the week prior to being randomly assigned to either vortioxetine or the melatonin receptor agonist/5-HT2B/2C antagonist agomelatine. Both agents could be titrated to their maximum recommended dosages. The researchers found that vortioxetine was statistically superior to agomelatine.
It is important to mention that three short-term trials examined cognitive function with a battery of tests, one in elderly patients and two with a primary outcome measure being improvement in cognitive function. All three trials yielded positive antidepressant action above placebo (Katona et al. 2012; Mahableshwarkar et al. 2015; McIntyre et al. 2014). Vortioxetine demonstrated a significantly greater effect than placebo on the Digit Symbol Substitution Task (DSST; a pencil-and-paper task that assesses attention, speed of processing, and executive function) in all three studies. In contrast, duloxetine (60 mg/day) showed no significant effect on the DSST versus placebo in the two studies that used this medication as a comparator (Katona et al. 2012; Mahableshwarkar et al. 2015). Furthermore, vortioxetine’s beneficial effect on cognition was deemed through path analysis to be mostly a direct effect that was independent of the drug’s antidepressant effect. One study used more routine daily tasks (the University of California San Diego Performance-Based Skills Assessment), and vortioxetine but not duloxetine separated from placebo, with the difference being almost entirely attributable to a direct effect (Mahableshwarkar et al. 2015). Taken together, the results of these studies led the European Medicines Agency, but not the FDA, to label vortioxetine for the treatment of cognitive dysfunction associated with major depressive disorder (McIntyre et al. 2016). There are, however, no studies reporting this putative benefit in patients with other diagnoses.
Side Effects and Toxicity
The main side effect of vortioxetine on treatment initiation is nausea, which occurs at about the same rate as with SSRIs and SNRIs, generally in about a quarter to a third of patients (Citrome 2014). This finding is somewhat surprising because vortioxetine is a potent 5-HT3 receptor antagonist, which should prevent any nausea resulting from 5-HT reuptake inhibition. However, nausea can be produced by a variety of chemical actions, including opioid and dopamine receptor activation and, importantly, 5-HT1A receptor agonism (as is the case with the 5-HT1A receptor agonist buspirone). The latter effect is likely responsible for the transient nausea reported during the initial 2 weeks of vortioxetine administration, given the drug’s potent 5-HT1A receptor agonism. This side effect led to treatment discontinuation in 1%–4% of subjects and appeared to be dose dependent (Baldwin et al. 2016).
Apart from gastrointestinal side effects, other side effects were generally not markedly different from those reported in the placebo group. Overall, discontinuation due to treatment-emergent adverse events was dose dependent and was between 4.5% and 8.5%, reaching the same level as with 60 mg/day of duloxetine, the placebo rate being 3.5% (Baldwin et al. 2016). When side effects are problematic, vortioxetine should be withheld for 3 days (given its long half-life) and restarted at half the previous daily dosage.
Vortioxetine has not been found to produce clinically meaningful effects on body weight in short-term and long-term clinical studies. It was not associated with an increased incidence of insomnia or daytime somnolence above the placebo level, in contrast to venlafaxine, which produced greater rates of insomnia, and duloxetine, which produced greater rates of somnolence. There are no significant discontinuation symptoms associated with abrupt cessation of vortioxetine, as expected from its long terminal half-life.
Sexual dysfunction is a classic side effect of drugs that potently inhibit the 5-HT transporter. For patients receiving vortioxetine 5 mg/day or 10 mg/day, rates of sexual dysfunction (as measured on the Arizona Sexual Experiences Scale) did not differ from rates for patients receiving placebo; however, for patients receiving vortioxetine 20 mg/day, rates of sexual dysfunction were equivalent to rates for patients receiving duloxetine 60 mg/day (Baldwin et al. 2016). These results are consistent with the lower occupancy of the 5-HT transporter with vortioxetine at dosages lower than 20 mg/day, which is about the same as with duloxetine 60 mg/day (i.e., 80%; Takano et al. 2006). In one study, 477 patients with major depressive disorder who were responding to paroxetine, sertraline, or citalopram but were experiencing treatment-emergent sexual dysfunction were switched to either vortioxetine or escitalopram (dosages for both drugs: 10 mg/day or 20 mg/day) (Jacobsen et al. 2015a). As measured by the Changes in Sexual Functioning Questionnaire Short Form (CSFQ-14), vortioxetine-treated patients showed significant improvements in comparison with escitalopram-treated patients on four of five dimensions and all three phases of sexual functioning assessed by the CSFQ-14. Although the absolute difference between the two groups was significant, it is important to note that three-quarters of the patients receiving vortioxetine were taking the higher dosage (20 mg/day).
No cases of vortioxetine overdose have been reported in the literature. A supratherapeutic dosage of 40 mg/day for 14 days did not produce clinically relevant electrocardiography or hemodynamic changes (i.e., QTc prolongation of 5 milliseconds) (Wang et al. 2013).
Drug–Drug Interactions
Vortioxetine must never be coadministered with a monoamine oxidase inhibitor (MAOI). Before a patient is started on vortioxetine, there must be an elimination period of 14 days for an irreversible MAOI or 2 days for the reversible MAOI moclobemide. Similarly, a 14-day elimination period for vortioxetine is necessary before initiating either an irreversible MAOI or moclobemide.
Vortioxetine is neither an inhibitor nor an inducer of any metabolic enzymes. Consequently, it will not alter the levels of other medications through metabolic interference. Complete inhibitors of CYP2D6, such as fluoxetine and paroxetine, as well as moderate inhibitors, such as bupropion and duloxetine, will approximately double the exposure (area under the curve) to vortioxetine. These interactions are expected to occur mainly in switch situations, which are discussed in the following section. In such concomitant regimens, the vortioxetine dosage should be reduced by half (D’Empaire et al. 2011; Hvenegaard et al. 2012).
Triptans used in the treatment of migraines may be less effective in the presence of vortioxetine. This class of agents acts mainly by activating 5-HT1D receptors, whereas vortioxetine is a 5-HT1D receptor antagonist. However, because vortioxetine’s affinity for the 5-HT1D receptor is very low compared with its affinities for other 5-HT receptor subtypes (Mørk et al. 2012), the dampened activity of triptans may not occur with lower dosages of vortioxetine.
Switching Strategies for Vortioxetine
Despite an extensive clinical program, there are only two studies reported on switching to vortioxetine from SSRIs and SNRIs (Jacobsen et al. 2015a; Montgomery et al. 2014). Although patients seemed to tolerate the transition to vortioxetine well, in clinical practice the switch process has been more problematic. It is likely that premature discontinuations of vortioxetine stem more from an abrupt, or a too-rapid, dosage decrease of the SSRI or SNRI than from the effects of vortioxetine itself (Rosenbaum and Zajecka 1997). As illustrated in Figure 16–2, SSRIs or SNRIs are eliminated within several days, thereby rapidly decreasing the occupancy of the 5-HT transporters, but vortioxetine steady-state plasma concentrations are achieved only after 2 weeks, producing a receptor occupancy of about 60% at 10 mg/day and 80% at 20 mg/day. Consequently, discontinuation symptoms could occur during a rapid switch that could be exacerbated by the potent 5-HT1A agonistic profile of vortioxetine. Therefore, implementation of a gradual crossover strategy is recommended when switching a patient from SSRIs or SNRIs to vortioxetine. One exception would be a switch from fluoxetine, because of its long half-life. However, because fluoxetine is a CYP2D6 inhibitor, the initial dosage of vortioxetine should be 5 mg/day, and the dosage should be gradually increased as fluoxetine is slowly eliminated.
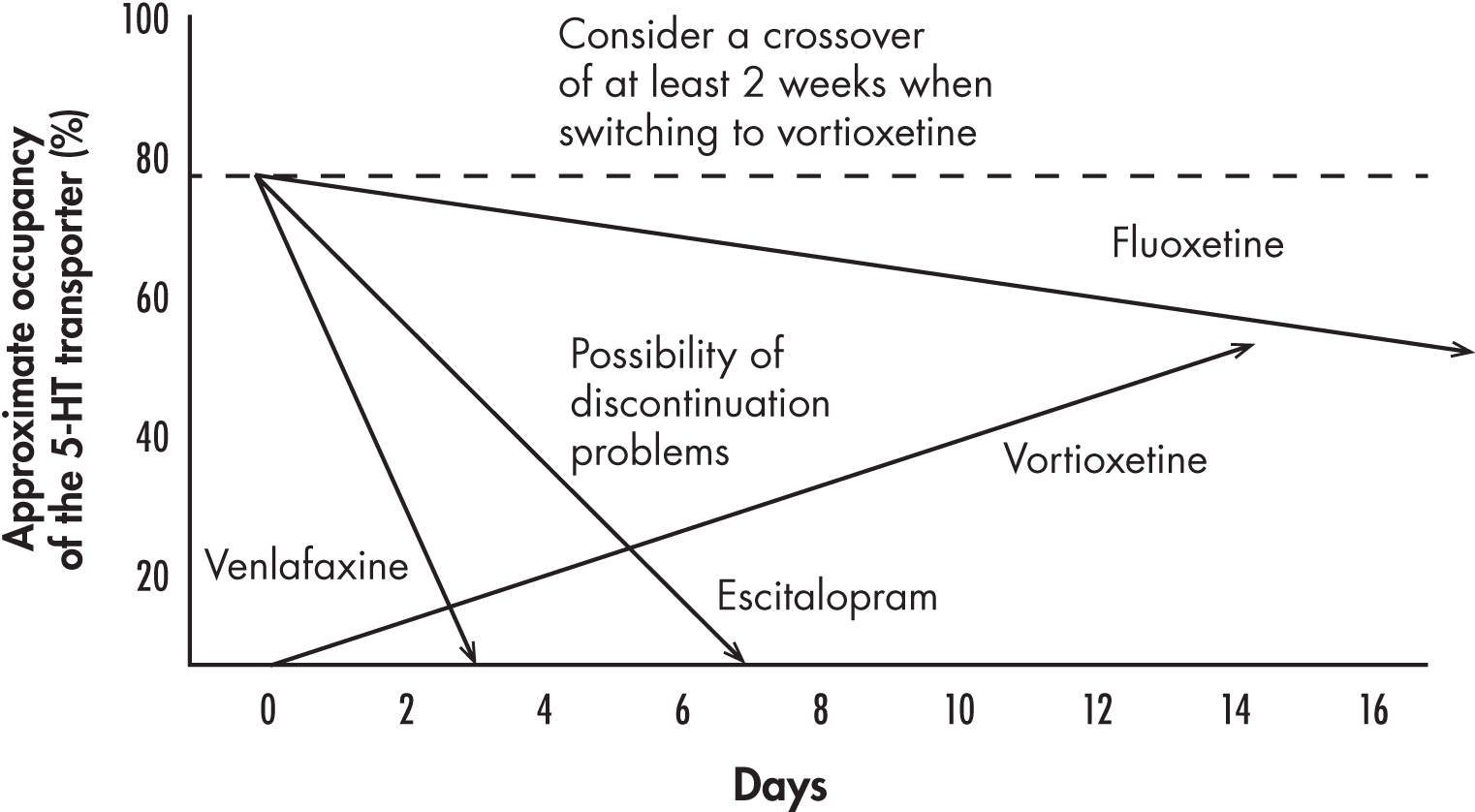
FIGURE 16–2. Relationship between occupancy of the serotonin transporter by various drugs used to treat depression and minimum time following their discontinuation and the introduction of vortioxetine.
The lines represent the decrease of serotonin (5-hydroxytryptamine [5-HT]) transporter occupancy following an abrupt cessation of the selective serotonin reuptake inhibitors escitalopram and fluoxetine and the serotonin–norepinephrine reuptake inhibitor venlafaxine in comparison with the rise of 5-HT transporter occupancy following the initiation of the multimodal agent vortioxetine at a dosage of 5–10 mg/day. When most 5-HT reuptake inhibitors (with the exception of fluoxetine) are discontinued abruptly before starting vortioxetine, discontinuation phenomena may be triggered, leading to an apparent intolerance of vortioxetine. Therefore, the previous medication should be gradually decreased while vortioxetine levels are building up.
Conclusion
Vortioxetine is a multimodal serotonergic agent that inhibits the 5-HT transporter, albeit to a lesser extent than other SSRIs, and has a variety of actions at five 5-HT receptor subtypes. Its only approved indication is for the treatment of major depressive disorder. Although it may produce as much mild to moderate nausea as SSRIs on treatment initiation, this effect is generally transient and seldom leads to treatment discontinuation. At dosages of 10 mg/day or less, vortioxetine is expected to produce less sexual dysfunction than SSRIs and SNRIs. Vortioxetine does not impact the cardiovascular system, even at twice its maximum recommended dosage. Vortioxetine will not alter the levels of other medications because it is not a hepatic enzyme inducer or inhibitor. It is still too early to determine vortioxetine’s role in treatment-resistant depression. However, in some patients with major depressive disorder, vortioxetine may exert a beneficial action on cognitive functioning.
References
Alvarez E, Perez V, Dragheim M, et al: A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol 15(5):589–600, 2012 21767441
Areberg J, Luntang-Jensen M, Søgaard B, et al: Occupancy of the serotonin transporter after administration of Lu AA21004 and its relation to plasma concentration in healthy subjects. Basic Clin Pharmacol Toxicol 110(4):401–404, 2012 21985522
Areberg J, Petersen KB, Chen G, Naik H: Population pharmacokinetic meta-analysis of vortioxetine in healthy individuals. Basic Clin Pharmacol Toxicol 115(6):552–559, 2014 24766668
Baldwin DS, Loft H, Dragheim M: A randomised, double-blind, placebo controlled, duloxetine-referenced, fixed-dose study of three dosages of Lu AA21004 in acute treatment of major depressive disorder (MDD). Eur Neuropsychopharmacol 22(7):482–491, 2012a 22209361
Baldwin DS, Loft H, Florea I: Lu AA21004, a multimodal psychotropic agent, in the prevention of relapse in adult patients with generalized anxiety disorder. Int Clin Psychopharmacol 27(4):197–207, 2012b 22475889
Baldwin DS, Chrones L, Florea I, et al: The safety and tolerability of vortioxetine: Analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol 30(3):242–252, 2016 26864543
Bang-Andersen B, Ruhland T, Jørgensen M, et al: Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem 54(9):3206–3221, 2011 21486038
Bidzan L, Mahableshwarkar AR, Jacobsen P, et al: Vortioxetine (Lu AA21004) in generalized anxiety disorder: results of an 8-week, multinational, randomized, double-blind, placebo-controlled clinical trial. Eur Neuropsychopharmacol 22(12):847–857, 2012 22898365
Boulenger JP, Loft H, Florea I: A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol 26(11):1408–1416, 2012 22495621
Boulenger JP, Loft H, Olsen CK: Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol 29(3):138–149, 2014 24257717
Bundgaard C, Eneberg E, Sánchez C: P-glycoprotein differentially affects escitalopram, levomilnacipran, vilazodone and vortioxetine transport at the mouse blood-brain barrier in vivo. Neuropharmacology 103:104–111, 2016 26700248
Chen G, Lee R, Højer AM, et al: Pharmacokinetic drug interactions involving vortioxetine (Lu AA21004), a multimodal antidepressant. Clin Drug Investig 33(10):727–736, 2013 23975654
Citrome L: Vortioxetine for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract 68(1):60–82, 2014 24165478
D’Empaire I, Guico-Pabia CJ, Preskorn SH: Antidepressant treatment and altered CYP2D6 activity: are pharmacokinetic variations clinically relevant? J Psychiatr Pract 17(5):330–339, 2011 21926528
Fu J, Peng L, Li X: The efficacy and safety of multiple doses of vortioxetine for generalized anxiety disorder: a meta-analysis. Neuropsychiatr Dis Treat 12(12):951–959, 2016 27143896
Henigsberg N, Mahableshwarkar AR, Jacobson P, Serenko M, et al: A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry 73(7):953–959, 2012 22901346
Hvenegaard MG, Bang-Andersen B, Pedersen H, et al: Identification of the cytochrome P450 and other enzymes involved in the in vitro oxidative metabolism of a novel antidepressant, Lu AA21004. Drug Metab Dispos 40(7):1357–1365, 2012 22496396
Jacobsen PL, Mahableshwarkar AR, Chen Y, et al: Effect of vortioxetine vs. escitalopram on sexual functioning in adults with well-treated major depressive disorder experiencing SSRI-induced sexual dysfunction. J Sex Med 12(10):2036–2048, 2015a 26331383
Jacobsen PL, Mahableshwarkar AR, Serenko M, et al: A randomized, double-blind, placebo-controlled study of the efficacy and safety of vortioxetine 10 mg and 20 mg in adults with major depressive disorder. J Clin Psychiatry 76(5):575–582, 2015b 26035185
Jain R, Mahableshwarkar AR, Jacobson PL, et al: A randomized, double-blind, placebo-controlled 6-wk trial of the efficacy and tolerability of 5 mg vortioxetine in adults with major depressive disorder. Int J Neuropsychopharmacol 16(2):313–321, 2013 22963932
Katona C, Hansen T, Olsen CK: A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol 27(4):215–223, 2012 22572889
Mahableshwarkar AR, Jacobson PL, Chen Y: A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin 29(3):217–226, 2013 23252878
Mahableshwarkar AR, Jacobson PL, Chen Y, et al: A randomized, double-blind, duloxetine-referenced study comparing efficacy and tolerability of 2 fixed doses of vortioxetine in the acute treatment of adults with MDD. Psychopharmacology (Berl) 232(12):2061–2070, 2015a 25575488
Mahableshwarkar AR, Jacobson PL, Serenko M, et al: A randomized, double-blind, placebo-controlled study of the efficacy and safety of 2 doses of vortioxetine in adults with major depressive disorder. J Clin Psychiatry 76(5):583–591, 2015b 26035186
Mahableshwarkar AR, Zajecka J, Jacobson W, et al: A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology 40(8):2025–2037, 2015c 25687662
McIntyre RS, Lophaven S, Olsen CK: A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol 17(10):1557–1567, 2014 24787143
McIntyre RS, Harrison J, Loft H, et al: The effects of vortioxetine on cognitive function in patients with major depressive disorder: a meta-analysis of three randomized controlled trials. Int J Neuropsychopharmacol Aug 24, 2016 [Epub ahead of print] 27312740
Meyer JH, Wilson AA, Sagrati S, et al: Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry 161(5):826–835, 2004 15121647
Montgomery SA, Nielsen RZ, Poulsen LH, et al: A randomised, double-blind study in adults with major depressive disorder with an inadequate response to a single course of selective serotonin reuptake inhibitor or serotonin-noradrenaline reuptake inhibitor treatment switched to vortioxetine or agomelatine. Hum Psychopharmacol 29(5):470–482, 2014 25087600
Mørk A, Pehrson A, Brennum LT, et al: Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther 340(3):666–675, 2012 22171087
Rosenbaum JF, Zajecka J: Clinical management of antidepressant discontinuation. J Clin Psychiatry 58 (suppl 7):37–40, 1997 9219493
Sanchez C, Asin KE, Artigas F: Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther 145:43–57, 2015 25016186
Stenkrona P, Halldin C, Lundberg J: 5-HTT and 5-HT(1A) receptor occupancy of the novel substance vortioxetine (Lu AA21004). A PET study in control subjects. Eur Neuropsychopharmacol 23(10):1190–1198, 2013 23428337
Takano A, Suzuki K, Kosaka J, et al: A dose-finding study of duloxetine based on serotonin transporter occupancy. Psychopharmacology (Berl) 185(3):395–399, 2006 16506079
Takeda Pharmaceuticals America: TRINTELLIX (vortioxetine) tablets, full prescribing information. Deerfield, IL, Takeda Pharmaceuticals America, Inc., 2016. Available at: http://general.takedapharm.com/content/file.aspx?filetypecode=BRINTELLIXPI&cacheRandomizer=59fe2b9b-08d3-44bb-a004-e6930f23abb3. Accessed November 2016.
Wang Y, Nomikos GG, Karim A, et al: Effect of vortioxetine on cardiac repolarization in healthy adult male subjects: results of a thorough QT/QTc study. Clin Pharmacol Drug Dev 2(4):298–309, 2013 27121934
Zohar J, Stahl S, Moller HJ, et al: A review of the current nomenclature for psychotropic agents and an introduction to the neuroscience-based nomenclature. Eur Neuropsychopharmacol 25(12):2318–2325, 2015 26527055