CHAPTER 28
Risperidone and Paliperidone
Michele Hill, M.R.C.Psych.
Donald C. Goff, M.D.
History and Discovery
A decade before clozapine was approved for marketing in the United States, Janssen Pharmaceuticals established a program to examine the potential role of serotonergic agents in schizophrenia. Early interest in serotonergic agents stemmed from preclinical literature demonstrating that both behavioral effects of dopamine receptor agonists and haloperidol-induced catalepsy could be modulated by serotonin 2 (5-HT2) receptor antagonists; in addition, the early butyrophenone derivative pipamperone, which was observed to reduce agitation and improve social activity in patients with severe depression, was found to possess primarily 5-HT2 receptor antagonist activity (Ansoms et al. 1977; Leysen et al. 1978).
In 1981, Janssen Pharmaceuticals developed setoperone, a 5-HT2 receptor antagonist with weak dopamine 2 (D2) receptor antagonism that displayed antipsychotic effects and efficacy for negative symptoms in a preliminary open trial (Ceulemans et al. 1985). Janssen Pharmaceuticals additionally synthesized a selective 5-HT2A and 5-HT2C receptor antagonist, ritanserin, which was shown to decrease extrapyramidal side effects (EPS) when combined with haloperidol in rat studies. Ritanserin also was active in animal models of anxiety (Colpaert et al. 1985; Meert and Colpaert 1986) and partially ameliorated the behavioral effects of lysergic acid diethylamide (LSD) (Colpaert et al. 1985). In placebo-controlled trials in patients with chronic schizophrenia, addition of ritanserin to first-generation antipsychotics (FGAs) improved negative symptoms and EPS (Bersani et al. 1990; Duinkerke et al. 1993; Gelders 1989; Reyntjens et al. 1986). Concluding that 5-HT2 receptor antagonism might improve the effectiveness of D2 blockers, particularly for negative symptoms, and reduce EPS, but that it was not sufficiently effective as monotherapy, Paul Janssen and colleagues undertook development of risperidone, which combined potent 5-HT2A and D2 receptor blockade.
After extensive preclinical characterization (Janssen et al. 1988), risperidone was first studied in clinical trials in 1986 and received U.S. Food and Drug Administration (FDA) approval for marketing in the United States in 1994. By the time risperidone became available to clinicians, the prominence of theories citing 5-HT2 enhancement of D2 receptor antagonism as a primary mechanism for clozapine’s atypical properties (Meltzer et al. 1989), and the evidence from registration trials of reduced EPS and greater efficacy compared with high-dose haloperidol, resulted in considerable enthusiasm for the first of the “serotonin–dopamine antagonists.” Risperidone was rapidly incorporated into clinical practice in the United States, where within 2 years it became the most frequently prescribed antipsychotic agent. In 2003, risperidone microspheres (Consta) received FDA approval as the first long-acting second-generation antipsychotic (SGA) designed for intramuscular (IM) injection. In December 2006, Janssen Pharmaceuticals introduced paliperidone (9-hydroxyrisperidone), the active metabolite of risperidone, formulated as an extended-release tablet marketed under the brand name Invega. Extended-release paliperidone is approved for the treatment of schizophrenia and schizoaffective disorder. A long-acting depot preparation, paliperidone palmitate (Sustenna), received FDA approval for schizophrenia in 2009 and for schizoaffective disorder in 2014; it was the first once-monthly depot formulation of an SGA to become available in the United States. In May 2015, a new formulation of paliperidone with a 3-month injection interval, paliperidone palmitate 3-month formulation (PP3M; Invega Trinza), was approved by the FDA for the treatment of schizophrenia under its priority review process. This formulation’s 3-month schedule provides the longest dosing interval available for patients with schizophrenia.
Pharmacological Profile
Risperidone, or 3-[2-(4-[6-fluoro-1,2-benzisoxazol-3-yl]-1-piperidinyl)ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one, is a benzisoxazole derivative characterized by very high affinity for 5-HT2A receptors and moderately high affinity for D2, histamine 1 (H1), and α1- and α2-adrenergic receptors (Figure 28–1). In vitro, the affinity of risperidone for 5-HT2A receptors is roughly 10- to 20-fold greater than that for D2 receptors (Leysen et al. 1994; Schotte et al. 1996); in vivo binding to rat striatal D2 receptors occurs at a dosage 10 times higher than does binding to 5-HT2A receptors (Leysen et al. 1994). The affinity for 5-HT2A receptors is more than 100-fold greater than for other serotonin receptor subtypes. Risperidone’s active metabolite 9-hydroxyrisperidone (paliperidone) (Figure 28–2) has a similar receptor affinity profile, although paliperidone has lower affinity for α1- and α2-adrenergic receptors. Both risperidone and paliperidone display a high affinity for 5-HT2A receptors in rat brain tissue and for cloned human receptors expressed in COS-7 cells (Leysen et al. 1994). Risperidone binds to 5-HT2A receptors with approximately 20-fold greater affinity compared with clozapine and 170-fold greater affinity compared with haloperidol (Leysen et al. 1994).
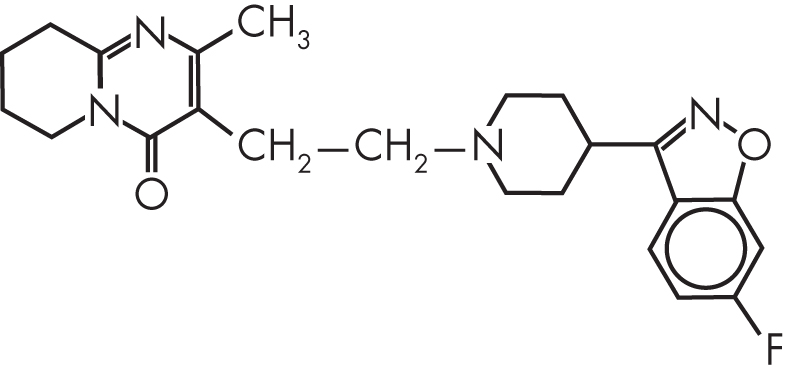
FIGURE 28–1. Chemical structure of risperidone.
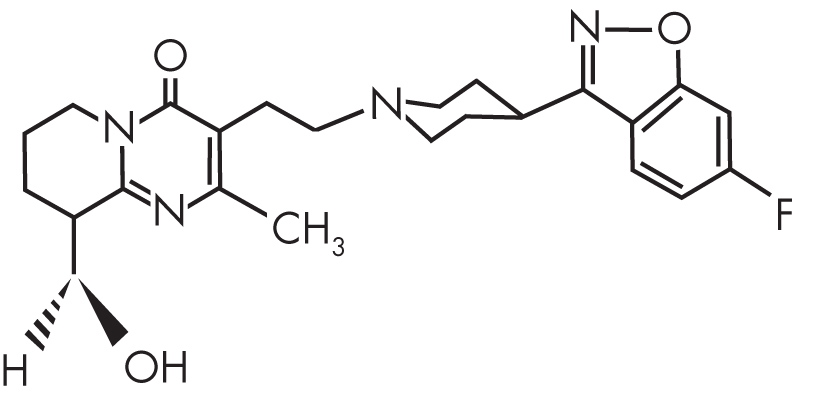
FIGURE 28–2. Chemical structure of paliperidone.
The affinity of risperidone for D2 receptors is approximately 50-fold greater than that of clozapine and approximately 20%–50% that of haloperidol (Leysen et al. 1994) (Table 28–1). Binding affinity for D2 receptors was similar in rat mesolimbic and striatal tissue and in the long and short forms of cloned human D2 receptors expressed in embryonic kidney cells (Leysen et al. 1993a). The affinities of risperidone and paliperidone for dopamine4 (D4) and dopamine1 (D1) receptors are similar to those of clozapine and haloperidol (Leysen et al. 1994). Risperidone and paliperidone have essentially no affinity for muscarinic acetylcholine receptors and modest H1 receptor activity. Unlike haloperidol, risperidone does not bind to sigma sites (Leysen et al. 1994). However, compared with other agents, including paliperidone, risperidone has a relatively high affinity for α2-adrenergic receptors that is substantially greater than that of clozapine or any FGA and that approaches the affinity of phentolamine (Richelson 1996). The affinity of risperidone for α1-adrenergic receptors is roughly comparable to that of chlorpromazine and approximately 5 to 10 times greater than that of clozapine (Leysen et al. 1993b; Richelson 1996). The median effective dose (ED50) of risperidone required to inhibit D2-mediated apomorphine-induced stereotypies in rats is 0.5 mg/kg; at this dose, approximately 40% of D2 receptors are occupied, as are 80% of 5-HT2A receptors, 50% of H1 receptors, 38% of α1-adrenergic receptors, and 10% of α2-adrenergic receptors (Leysen et al. 1994).
Paliperidone |
Risperidone |
Haloperidol |
Clozapine |
|
Dopaminergic |
||||
D2a |
4.8 |
5.9 |
2.2 |
190 |
D1a |
670 |
620 |
270 |
540 |
Serotonergic |
||||
5-HT2Aa |
1.0 |
0.52 |
200 |
9.6 |
5-HT1Aa |
590 |
420 |
1,500 |
140 |
Adrenergic |
||||
α1b |
4.0 |
2.3 |
19 |
23 |
α2b |
17 |
7.5 |
>5,000 |
160 |
H1 histaminergica |
32 |
27 |
790 |
0.23 |
Muscarinicb |
3,570 |
>5,000 |
4,670 |
34 |
aCloned human receptor. bRat brain. Source. Adapted from Schotte et al. 1996. |
||||
Several groups have studied the occupancy of D2 and 5-HT2 receptors in patients with schizophrenia, employing positron emission tomography (PET) or single-photon emission computed tomography (SPECT) ligand-binding techniques. Kapur et al. (1999) used PET to measure D2 occupancy with 11C-labeled raclopride and 5-HT2 occupancy with 18F-labeled setoperone in patients with chronic schizophrenia maintained on a stable clinician-determined dose of risperidone. The PET was performed 12–14 hours after the last dose of risperidone. Occupancy of D2 receptors ranged from 63% to 89%; 50% occupancy was calculated to occur with a daily risperidone dose of 0.8 mg. Patients treated with risperidone (6 mg/day) exhibited a mean D2 occupancy of 79%, which was consistent with the mean occupancy of 82% that was previously reported by Nyberg et al. (1999) and would be expected to exceed the putative threshold for EPS in some patients. A similar degree of D2 occupancy was calculated to occur with olanzapine at approximately 30 mg daily (Kapur et al. 1999). A maximal 5-HT2 occupancy of greater than 95% was achieved with risperidone at daily doses as low as 2–4 mg. In a small sample of patients treated biweekly for at least 10 weeks with risperidone microspheres (Consta), Remington et al. (2006) found that the 25-mg dose produced a mean D2 occupancy of 54% (preinjection) and 71% (postinjection), whereas the 50-mg dose produced occupancy levels of 65% (preinjection) and 74% (postinjection). Arakawa et al. (2008) found a D2 occupancy of 58% with paliperidone at 3 mg/day and 77% with 9 mg/day, consistent with clinical estimates that paliperidone is roughly half as potent as risperidone, perhaps due to reduced bioavailability (de Leon et al. 2010).
Preclinical characterization of risperidone in rats revealed more potent antiserotonergic activity compared with ritanserin in all tests (Janssen et al. 1988). For example, in reversal of tryptophan-induced effects in rats, risperidone was 6.4 times more potent than ritanserin for reversal of peripheral 5-HT2-mediated effects and 2.4 times more potent for reversal of centrally mediated 5-HT2 effects (Janssen et al. 1988). Risperidone was also found to completely block discrimination of LSD, in contrast to the partial attenuation observed with ritanserin (Meert et al. 1989). Although risperidone demonstrated activity in all dopamine-mediated tests, the dose–response pattern differed from that of haloperidol (Janssen et al. 1988). The two drugs were roughly equipotent for inhibition of certain dopamine effects, such as amphetamine-induced oxygen hyperconsumption, whereas the dose of risperidone necessary to cause pronounced catalepsy in rats was 18-fold higher than that of haloperidol (Janssen et al. 1988). Risperidone depressed vertical and horizontal activity in rats at a dose 2–3 times greater than that of haloperidol but required doses more than 30 times greater than those of haloperidol to depress small motor movements (Megens et al. 1988).
Pharmacokinetics and Disposition
Risperidone is rapidly absorbed after oral administration, with peak plasma levels achieved within 1 hour (Heykants et al. 1994). In early Phase I studies, risperidone demonstrated linear pharmacokinetics at dosages between 0.5 and 25 mg/day (Mesotten et al. 1989; Roose et al. 1988).
After a single dose of the extended-release formulation of paliperidone (Invega), serum concentrations gradually increase until a maximum concentration is achieved approximately 24 hours after ingestion. Absorption of paliperidone is increased by approximately 50% when taken with a meal compared with the fasting state. Extended-release paliperidone also demonstrates dose-proportional pharmacokinetics within the recommended dosing range (3–12 mg/day).
Because risperidone lacks a hydroxyl group to which an ester can be bound for a traditional oil-based depot preparation, polymer microsphere technology was used to produce a slow-release injectable formulation. Risperidone microspheres do not begin to release appreciable amounts of drug until 3 weeks after injection and continue to release drug for approximately 4 weeks, with maximal drug release occurring after about 5 weeks.
Paliperidone palmitate is an esterified form of paliperidone and is poorly soluble in water. It dissolves slowly following IM injection, after which it is hydrolyzed to active paliperidone and absorbed into the systemic circulation. Systemic availability of paliperidone begins on day 1 and can last as long as 126 days after injection. Maximum blood levels are achieved a median of 13 days after injection. A dose of 75 mg/month is recommended, but in practice the modal dose is 100 mg/month (Attard et al. 2014). IM injection in the deltoid produces blood levels approximately 28% higher than those produced with gluteal injection. The 3-month formulation of paliperidone palmitate has an extended apparent elimination half-life that permits this longer dosing interval. Risperidone is 90% plasma protein bound, whereas paliperidone is 74% plasma protein bound (Borison et al. 1994). The absolute bioavailability of risperidone is about 100%; that of extended-release paliperidone is about 28%. Paliperidone may achieve relatively lower concentrations in the brain because of its higher affinity for the extruding P-glycoprotein transporter, which limits the amount of drug crossing the blood–brain barrier (de Leon et al. 2010).
Risperidone is metabolized by hydroxylation of the tetrahydropyridopyrimidinone ring at the seven and nine positions and by oxidative N-dealkylation (Mannens et al. 1993). The most important metabolite, 9-hydroxyrisperidone (paliperidone), accounts for up to 31% of the dose excreted in the urine. Because hydroxylation of risperidone is catalyzed by cytochrome P450 (CYP) 2D6, the half-life of the parent compound varies according to the relative activity of this enzyme. In extensive metabolizers, which include about 90% of Caucasians and as many as 99% of Asians, the half-life of risperidone is approximately 3 hours. Approximately 60% of paliperidone is excreted unchanged in the urine, and the remainder is metabolized by at least four different pathways (dealkylation, hydroxylation, dehydrogenation, and benzisoxazole scission), none of which accounts for more than 10% of the total. The terminal half-life of 9-hydroxyrisperidone (and of extended-release paliperidone) is 23 hours. Poor metabolizers metabolize risperidone primarily via oxidative pathways; the half-life may exceed 20 hours. In extensive metabolizers, radioactivity from 14C-labeled risperidone is not detectable in plasma 24 hours after a single dose, whereas 9-hydroxyrisperidone accounts for 70%–80% of radioactivity. In poor metabolizers, risperidone is primarily responsible for radioactivity after 24 hours. In the U.S. multicenter registration trial, the correlations between risperidone dose and serum risperidone and 9-hydroxyrisperidone concentrations were 0.59 and 0.88, respectively (Anderson et al. 1993). Because of paliperidone’s longer half-life, its serum concentrations at steady state are approximately 5- to 10-fold higher than risperidone concentrations in CYP2D6 extensive metabolizers treated with risperidone (de Leon et al. 2010). However, in 2D6 poor metabolizers and patients concurrently taking 2D6 inhibitors, risperidone concentrations may be higher than paliperidone concentrations.
Mechanism of Action
As previously discussed, risperidone was developed specifically to exploit the apparent pharmacological advantages of combining 5-HT2 receptor antagonism with D2 receptor blockade. Selective 5-HT2A receptor antagonists administered alone have demonstrated activity in several animal models suggestive of antipsychotic effect, including blockade of both amphetamine- and phencyclidine (PCP)-induced locomotor activity (Schmidt et al. 1995). Dizocilpine-induced disruption of prepulse inhibition is also blocked by 5-HT2A receptor antagonists, suggesting that sensory gating deficits characteristic of schizophrenia and perhaps resulting from glutamatergic dysregulation might also benefit from the 5-HT2 receptor antagonism of risperidone (Varty et al. 1999). The disruption of prepulse inhibition by dizocilpine (MK-801, a noncompetitive N-methyl-D-aspartate [NMDA] receptor antagonist) is attenuated by SGAs, but not by first-generation D2 receptor blockers (Geyer et al. 1990). From a study in which the selective 5-HT2A receptor antagonist M100907 was added to low-dose raclopride (a selective D2 receptor blocker), Wadenberg et al. (1998) concluded that 5-HT2A antagonism facilitates D2 receptor antagonist blockade of conditioned avoidance, another behavioral model associated with antipsychotic efficacy, but does not block conditioned avoidance when administered alone.
One mechanism by which risperidone, paliperidone, and similar atypical agents might produce enhanced efficacy for negative symptoms and cognitive deficits and reduced risk for EPS is via 5-HT2A receptor modulation of dopamine neuronal firing and cortical dopamine release. Prefrontal dopaminergic hypoactivity has been postulated to underlie negative symptoms and cognitive deficits in schizophrenia (Goff and Evins 1998); both clozapine and ritanserin have been shown to increase dopamine release in prefrontal cortex, whereas haloperidol does not (Busatto and Kerwin 1997). Following 21 days of administration, risperidone, but not haloperidol, continued to increase dopamine turnover in the dorsal striatum and prefrontal cortex (Stathis et al. 1996). Ritanserin has been shown to enhance midbrain dopamine cell firing by blocking a tonic inhibitory serotonin input (Ugedo et al. 1989). Ritanserin also normalized ventral tegmental dopamine neuron firing patterns in rats after hypofrontality was induced by experimental cooling of the frontal cortex (Svensson et al. 1989).
Svensson et al. (1995) performed a series of elegant studies examining the impact of atypical antipsychotics on ventral tegmental dopamine firing patterns disrupted by glutamatergic NMDA receptor antagonists. In healthy human subjects, administration of the NMDA antagonist ketamine is widely regarded as a promising model for several clinical aspects of schizophrenia, including psychosis, negative symptoms, and cognitive deficits (Goff and Coyle 2001; Krystal et al. 1994). In rats, administration of the NMDA channel blockers dizocilpine or PCP increased burst firing of ventral tegmental dopamine neurons predominantly projecting to limbic structures but reduced firing of mesocortical tract dopamine neurons and disrupted firing patterns. Administration of ritanserin or clozapine preferentially enhanced firing of dopamine neurons with cortical projections, and when added to a D2 blocker, ritanserin increased dopamine release in prefrontal cortex. In addition to modulating ventral tegmental dopamine neuron firing, risperidone also blocks 5-HT2 receptors on inhibitory γ-aminobutyric acid (GABA)-ergic interneurons, which could also influence activity of cortical pyramidal neurons that are regulated by these local inhibitory circuits (Gellman and Aghajanian 1994).
In placebo-controlled clinical trials, 5-HT2 receptor antagonists have shown efficacy in reducing antipsychotic-induced parkinsonism and akathisia (Duinkerke et al. 1993; Poyurovsky et al. 1999). This effect may reflect 5-HT2A antagonist effects on nigrostriatal dopamine release. When combined with haloperidol, selective 5-HT2 receptor antagonists increase dopamine metabolism in the striatum and prevent an increase in D2 receptor density, thereby possibly reducing the effects of D2 receptor blockade and dopamine supersensitivity (Saller et al. 1990). These agents do not affect dopamine metabolism in the absence of D2 blockade.
The relative importance of 5-HT2 receptor antagonist activity in producing atypical characteristics is the subject of debate. As argued by Kapur and Seeman (2001) and Seeman (2002), most SGAs have dissociation constants for the D2 receptor that are larger than the dissociation constant of dopamine. This “loose binding” to the D2 receptor may allow displacement by endogenous dopamine and may contribute to a reduced liability for EPS and hyperprolactinemia. Unique among atypical agents, risperidone is “tightly bound” to the D2 receptor, with a dissociation constant smaller than that of dopamine (Seeman 2002). A model for atypical antipsychotic mechanisms that emphasizes D2 dissociation constants would predict that the apparent atypicality of risperidone, compared with that of haloperidol, reflects the reduced D2 receptor occupancy achieved by more favorable dosing rather than the intrinsic pharmacological characteristics of risperidone. According to some binding data, a comparable clinical dosage of haloperidol would be approximately 4 mg/day, rather than 20 mg/day as used in the North American multicenter registration trial (Kapur et al. 1999). Consistent with this view, benefits of risperidone for negative symptoms and EPS were less apparent when compared with lower doses of haloperidol or with lower-potency FGAs (see “Indications and Efficacy” section later in this chapter) than when compared with high-dose haloperidol (20 mg/day).
An additional mechanism possibly contributing to the enhanced efficacy of risperidone and paliperidone is their considerable α-adrenergic receptor antagonism. In a placebo-controlled augmentation trial, Litman et al. (1996) demonstrated significant improvement in psychosis and negative symptoms with the α2-adrenergic receptor antagonist idazoxan when it was added to FGAs. Idazoxan has been shown to increase dopamine levels in the rat medial prefrontal cortex (Hertel et al. 1999). In aged rats (Haapalinna et al. 2000) and in patients with frontal dementias (Coull et al. 1996), α2-adrenergic receptor blockers have also been reported to improve cognitive functioning. Svensson et al. (1995) found that prazosin, an α1 receptor antagonist, inhibited both the behavioral activation and the increase in mesolimbic dopamine release produced by PCP or MK-801.
In summary, risperidone and paliperidone possess at least two mechanisms that may confer atypical characteristics. 5-HT2A receptor antagonism partially protects against D2 antagonist–induced neurological side effects and may improve negative symptoms and cognitive functioning via modulation of mesocortical dopamine activity. In addition, blockade of adrenoceptors may further increase prefrontal cortical activity and could enhance antipsychotic efficacy by modulation of mesolimbic dopamine activity. Unlike other SGA agents, risperidone and paliperidone do not differ from FGAs in their dissociation constant for the D2 receptor; this feature perhaps accounts for the risk of EPS at high doses, as well as their greater propensity to cause hyperprolactinemia.
Indications and Efficacy
Risperidone is approved by the FDA for the treatment of schizophrenia, bipolar mania, and irritability associated with autism. In August 2007, the indication for schizophrenia was extended to include adolescents ages 13–17 years, and the bipolar mania indication was extended to include children 10–17 years of age. The risperidone microsphere formulation (Consta long-acting injectable [LAI]) is approved for the treatment of schizophrenia and bipolar I disorder. Extended-release oral paliperidone (Invega) is approved for the treatment of schizophrenia and schizoaffective disorder, LAI paliperidone palmitate (Sustenna) is approved for the treatment of schizophrenia and schizoaffective disorder, and paliperidone palmitate 3-month injection formulation (Invega Trinza) is approved for the treatment of schizophrenia.
Schizophrenia
Clinical Trial Results for Risperidone
Eight-week trials. In the two North American registration trials (Chouinard et al. 1993; Marder and Meibach 1994), a total of 513 patients with chronic schizophrenia were randomly assigned to an 8-week double-blind, fixed-dose, placebo-controlled comparison of risperidone (2, 6, 10, or 16 mg/day) versus haloperidol (20 mg/day). Risperidone dosages of 6, 10, and 16 mg/day produced significantly greater reductions, as compared with haloperidol, in each of the five domains of the Positive and Negative Syndrome Scale (PANSS), derived by principal-components analysis (Marder et al. 1997), and significantly higher response rates, defined as a 20% reduction in the PANSS total score. Effect sizes representing the difference in change scores between risperidone (6 mg/day) and haloperidol, although statistically significant, were uniformly small by Cohen’s classification system (Cohen 1988): negative symptoms 0.31; positive symptoms 0.26; disorganized thoughts 0.22; uncontrolled hostility/excitement 0.29; and anxiety/depression 0.30 (Table 28–2). Severity of EPS was greater with haloperidol than with risperidone; further statistical analysis suggested that differences in EPS rates did not significantly influence the differences in PANSS subscale ratings (Marder et al. 1997). In fact, risperidone 10 mg/day and 16 mg/day produced improvements in negative symptoms equivalent to those seen with risperidone 6 mg/day, despite increased EPS at the higher dosages of risperidone.
Adjusted mean change scores |
Risperidone 6 mg/day |
|||||
Placebo |
Risperidone 6 mg/day |
Haloperidol 20 mg/day |
Effect size vs. placebo |
Effect size vs. haloperidol |
||
PANSS total |
−3.8 |
−18.6 |
−5.1 |
0.53 |
0.31 |
|
Negative |
0.2 |
−3.4 |
−0.1 |
0.27 |
0.26 |
|
Positive |
0.9 |
−5.7 |
−2.3 |
0.48 |
0.22 |
|
Disorganized thought |
0.1 |
−4.6 |
−0.2 |
0.43 |
0.24 |
|
Hostility/excitement |
0.2 |
−2.5 |
−0.1 |
0.47 |
0.29 |
|
Anxiety/depression |
−0.1 |
−2.5 |
−0.6 |
0.36 |
0.30 |
|
Source. Adapted from Marder et al. 1997. |
||||||
When risperidone (1, 4, 8, 12, and 16 mg/day) was compared with haloperidol (10 mg/day) in a large 8-week European trial involving 1,362 subjects with schizophrenia (Peuskens 1995), PANSS subscale change scores among risperidone-treated subjects indicated a preferential response to daily doses of 4 mg and 8 mg. However, neither the risperidone group taken as a whole nor individual risperidone doses achieved significantly better outcomes than haloperidol (10 mg/day) on any measure except for EPS, suggesting that the clinical superiority of risperidone over haloperidol in previous studies may have resulted from excessively high dosing of the comparator.
CATIE. In the National Institute of Mental Health–funded Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE; Stroup et al. 2003), 1,432 patients with chronic schizophrenia were randomly assigned to double-blind, flexibly dosed treatment for 18 months with risperidone, olanzapine, quetiapine, ziprasidone, or the FGA comparator perphenazine. Clinicians could adjust the dosage of each drug by prescribing 1–4 capsules daily; risperidone capsules contained 1.5 mg, and the mean daily dose administered in the study was 3.9 mg. Based on the primary outcome measure, time to all-cause discontinuation, risperidone was less effective than olanzapine (mean dosage 20 mg/day) and comparable in effectiveness to perphenazine, quetiapine, and ziprasidone (Lieberman et al. 2005). Although differences in rates of dropout due to intolerance did not reach statistical significance, risperidone consistently was the best-tolerated drug, particularly in subjects who had discontinued their first-assigned drug due to intolerance.
Maintenance treatment. Csernansky et al. (2002) randomly assigned 365 patients with stable schizophrenia or schizoaffective disorder to clinician-determined flexible dosing with risperidone or haloperidol for a minimum of 1 year. Kaplan-Meier estimates of the risk of relapse at the end of the study were 34% with risperidone versus 60% with haloperidol, a highly significant difference (P=0.001). The LAI risperidone microsphere formulation (Consta) at fixed doses of 25 mg, 50 mg, and 75 mg administered biweekly was superior in efficacy to placebo in a 12-week trial (Kane et al. 2003), but the 75-mg dose was not developed further due to an increased rate of EPS. In a 52-week maintenance study comparing fixed doses of risperidone microspheres administered every 2 weeks, the 25-mg dose was associated with a relapse rate of 21.6% and the 50-mg dose was associated with a 14.9% relapse rate (P=0.06) (Simpson et al. 2006). Comparisons of risperidone microspheres against oral SGAs have failed to demonstrate greater benefit with the LAI formulation. In 12-month randomized trials, the efficacy of risperidone microspheres was similar to that of oral risperidone (n=50; Bai et al. 2007), oral olanzapine (n=377; Keks et al. 2007), or clinician’s choice of an oral antipsychotic other than clozapine (n=369; Rosenheck et al. 2011). By contrast, a nationwide cohort study using national databases in Norway found risperidone microspheres to be more effective in preventing readmission following a first hospitalization compared with oral risperidone and other oral antipsychotics with the exception of olanzapine and clozapine (Tiihonen et al. 2011). Because antipsychotic treatment in this naturalistic study was not randomized, conclusions are limited; nonetheless, the advantage found with depot formulations may reflect performance under typical clinical conditions.
Cognitive functioning in schizophrenia. Several early studies suggested that risperidone might enhance cognitive functioning, particularly verbal working memory, compared with haloperidol (Green et al. 1997; Harvey et al. 2005). A large double-blind trial that examined cognitive effects in 414 chronic schizophrenia patients treated for 52 weeks with risperidone (mean dosage 5.2 mg/day), olanzapine (12.3 mg/day), and haloperidol (8.2 mg/day) found no difference between treatments on the composite cognitive score, although risperidone and olanzapine were superior to haloperidol in a secondary analysis of completers (Keefe et al. 2006). When compared with low-dose haloperidol (mean dosage 5 mg/day), no cognitive advantage was found for risperidone (Green et al. 2002). In addition, in the CATIE, neither risperidone nor any other SGA demonstrated cognitive benefit compared with the FGA agent perphenazine (Keefe et al. 2007). A study in medication-naive patients found similar overall cognitive improvement with risperidone and olanzapine (Cuesta et al. 2009); however, another study of risperidone in medication-naive patients reported impairments of spatial working memory (Reilly et al. 2006) and procedural memory (Harris et al. 2009).
First-episode and treatment-refractory schizophrenia. Risperidone has been found to be well tolerated and effective in subgroups of patients with schizophrenia, including first-episode patients and elderly patients. In a 4-month double-blind trial comparing risperidone (mean dosage 3.9 mg/day) and olanzapine (mean dosage 11.8 mg/day) in 112 first-episode patients, both treatments were well tolerated, with an overall completion rate of 72% (Robinson et al. 2006). Response rates did not differ significantly between risperidone (54%) and olanzapine (44%), although patients who responded to risperidone were significantly more likely to retain their response. A recent 12-month double-blind trial comparing oral risperidone with LAI risperidone in 86 patients with recent-onset schizophrenia showed significantly lower relapse rates in the patients receiving the depot formulation (Subotnik et al. 2015). However, another randomized controlled trial of 85 first-episode patients found oral SGAs and LAI risperidone to be equally effective and to have similar safety profiles (Malla et al. 2013). This finding implies that LAI risperidone is a safe, effective treatment option in this population, although not necessarily the treatment of first choice.
Experience in patients with treatment-resistant schizophrenia has been less consistent. In the U.S. multicenter registration study, Marder and Meibach (1994) found that patients who were presumed to have failed to respond to FGAs, on the basis of a history of hospitalization for at least 6 months prior to study entry, did not respond to haloperidol (20 mg/day) but did display significant response to risperidone (6 mg/day and 16 mg/day) compared with placebo. Wirshing et al. (1999) reported significant improvement with risperidone (6 mg/day) compared with haloperidol (15 mg/day) during a 4-week fixed-dose trial in 67 patients with schizophrenia and a history of treatment resistance. In contrast, Volavka et al. (2002) found no difference between high-dose risperidone (8–16 mg/day) and haloperidol (10–20 mg/day) in patients established by history to be treatment resistant to FGAs. In the CATIE, risperidone was more effective than quetiapine but did not differ from olanzapine and ziprasidone in patients who discontinued their first-assigned SGA medication due to lack of efficacy (Stroup et al. 2006). In contrast, patients who discontinued perphenazine (for any reason) subsequently did better on quetiapine or olanzapine than they did on risperidone (Stroup et al. 2007). Two meta-analyses comparing FGAs and SGAs suggested an advantage for risperidone over older drugs (Davis et al. 2003; Leucht et al. 2009), and a recent network meta-analysis broadly confirmed this suggestion (Leucht et al. 2013).
Clinical Trial Results for Paliperidone
In a 6-week trial in acutely ill schizophrenia patients, extended-release paliperidone (Invega) at dosages of 6, 9, and 12 mg/day was more effective than placebo (Kane et al. 2007), and in a flexibly dosed trial (9–15 mg/day), extended-release paliperidone significantly reduced relapse compared with placebo (Kramer et al. 2007). Combined results from three placebo-controlled trials involving 1,326 acutely ill schizophrenia patients found significant improvement across a daily dosage range of 3–15 mg (Meltzer et al. 2008). Three studies compared paliperidone with olanzapine 10 mg/day and found no difference in efficacy, but paliperidone was associated with more movement disorders and less weight gain (Nussbaum and Stroup 2008). The network meta-analysis by Leucht et al. (2013) concluded that paliperidone was significantly less effective than clozapine, amisulpride, and olanzapine but not risperidone, although unlike risperidone, paliperidone failed to demonstrate superiority over older drugs.
LAI paliperidone palmitate administered every 4 weeks has been demonstrated to be more effective than placebo (Hough et al. 2010; Kramer et al. 2010; Nasrallah et al. 2010; Takahashi et al. 2013) and to be similar in efficacy to risperidone microspheres administered every 2 weeks (Pandina et al. 2011), although the latter finding was not replicated in another study (Fleischhacker et al. 2012). A randomized clinical trial comparing LAI paliperidone palmitate with depot haloperidol administered in a matched loading-dose schedule (McEvoy et al. 2014) showed that the two drugs were equally effective in preventing relapse.
A Phase III clinical trial of paliperidone palmitate 3-month formulation (Invega Trinza) versus placebo was terminated early after the interim analysis clearly demonstrated the superior efficacy of PP3M (hazard ratio=3.45; 95% confidence interval=1.73–6.88; P<0.001) (Berwaerts et al. 2015).
A second Phase III randomized, double-blind noninferiority study (N=1,016) comparing PP3M with paliperidone palmitate 1-month formulation (PP1M) found no differences in efficacy, relapse rates, or side-effect profiles between the two formulations (Savitz et al. 2016).
Mood Disorders
Six controlled trials of 3–4 weeks’ duration that included a total of 1,343 patients have examined the efficacy of risperidone as monotherapy or in combination with a mood stabilizer for the acute treatment of bipolar mania (Rendell et al. 2006). As monotherapy and in combination, risperidone was more effective than placebo and similar in efficacy to haloperidol but produced more weight gain and fewer EPS (Rendell et al. 2006). In a placebo-controlled trial of open-label risperidone LAI microspheres in the maintenance treatment of bipolar I disorder, patients (n=303) with manic or mixed episodes who maintained response during a preceding 26-week period of risperidone following an initial 3-week period of oral risperidone were randomly allocated to placebo injections or continued treatment with LAI risperidone for up to 24 months. A switch to placebo injections significantly shortened the time to recurrence of manic episodes, but not depressive episodes (Quiroz et al. 2010).
Risperidone 1–2 mg/day was evaluated as an adjunct to antidepressant therapy in a 4-week placebo-controlled trial in 174 antidepressant-resistant patients with major depressive disorder recruited from 19 primary care and psychiatric centers (Mahmoud et al. 2007). Risperidone significantly lowered ratings of depressive symptoms compared with placebo. Remission rates were 25% with risperidone versus 11% with placebo (P=0.004). Risperidone was well tolerated, with an 81% completion rate (vs. 88% with placebo).
Autism Spectrum Disorder
Risperidone also was studied in a large 8-week placebo-controlled trial in 101 children and adolescents (ages 5–17 years) with DSM-IV (American Psychiatric Association 1994)–defined autistic disorder accompanied by severe tantrums, aggression, or self-injurious behavior (McCracken et al. 2002). Flexible dosing with risperidone (range=0.5–3.5 mg/day; mean dosage=1.2 mg/day) resulted in a mean reduction of 57% in irritability, compared with a decrease of 14% in the placebo group, and the response rate was 69% with risperidone versus 12% with placebo. In a study of 32 children (ages 5–17 years) with DSM-IV autistic disorder who were treated for 4 months with open-label risperidone (mean dosage 2 mg/day), those who continued treatment with risperidone during the second study arm, an 8-week double-blind substitution trial, had much lower relapse rates compared with patients who were switched to placebo (Research Units on Pediatric Psychopharmacology Autism Network 2005). Risperidone at a mean dosage of 2 mg/day was also found to be effective compared with placebo in a study of 31 adults with DSM-IV autistic disorder or pervasive developmental disorder not otherwise specified (McDougle et al. 1998). In these studies, risperidone improved irritability and restricted, repetitive, and stereotyped behavioral problems associated with autism but was not effective for social or language deficits (McDougle et al. 2005). Risperidone at a dosage of 0.02–0.06 mg/kg was found to be well tolerated and effective for disruptive behaviors in children with low intelligence (IQ between 36 and 84) in a 6-week placebo-controlled trial (Aman et al. 2002).
Other Disorders
Generalized Anxiety Disorder
In a 4-week placebo-controlled add-on trial of low-dose risperidone in 417 patients with persistence of generalized anxiety disorder symptoms despite 8 weeks of anxiolytic therapy, no benefit from risperidone was found in the primary analysis; however, risperidone was associated with greater improvement in patients with moderate or severe anxiety at baseline (Pandina et al. 2007). Risperidone was highly effective for obsessive-compulsive disorder symptoms in a 6-week placebo-controlled trial in 36 adults prospectively confirmed to be nonresponsive to treatment with a selective serotonin reuptake inhibitor (McDougle et al. 2000). Symptoms of anxiety and depression also responded to risperidone compared with placebo. Fifty percent of risperidone-treated patients responded (mean dosage 2.2 mg/day), compared with none in the placebo group.
Alzheimer’s Disease
In the CATIE–Alzheimer’s Disease (CATIE-AD) study, risperidone had the longest time to discontinuation due to lack of effectiveness (27 weeks) among the agents studied; comparison results were 22 weeks for olanzapine, 9 weeks for quetiapine, and 9 weeks for placebo (Schneider et al. 2006). However, because of poor tolerability, none of the three antipsychotics differed from placebo on time to all-cause discontinuation.
Side Effects and Toxicology
Risperidone shares class warnings with other SGAs in the United States, including the risks of tardive dyskinesia, neuroleptic malignant syndrome, and hyperglycemia and diabetes, as well as the risk of increased mortality in elderly patients with dementia-related psychosis. However, risperidone generally has been very well tolerated in clinical trials. In the U.S. multicenter trial reported by Marder and Meibach (1994), only headache and dizziness were significantly more frequent with risperidone (6 mg/day) compared with placebo, whereas the group receiving risperidone (16 mg/day) treatment also reported more EPS and dyspepsia than did the group receiving placebo (Table 28–3). Fatigue, sedation, accommodation disturbances, orthostatic dizziness, palpitations or tachycardia, weight gain, diminished sexual desire, and erectile dysfunction displayed a statistically significant relationship to risperidone dose, although most were not significantly elevated compared with placebo. In a flexible-dose relapse prevention study reported by Csernansky et al. (2002), no side effects were more frequent with risperidone, compared with haloperidol, although risperidone produced significantly greater weight gain. In a flexibly dosed, placebo-controlled trial of risperidone for children with disruptive behavior, risperidone (mean dosage 1.2 mg/day) produced more somnolence, headache, vomiting, dyspepsia, weight gain, and prolactin elevation than did placebo; most side effects were rated mild to moderate and did not adversely affect compliance (Aman et al. 2002).
Percentage of patients |
|||||
Placebo (n=66) |
Risperidone 6 mg (n=64) |
Risperidone 16 mg (n=64) |
Haloperidol (n=66) |
||
Insomnia |
9.1 |
12.5 |
9.4 |
12.1 |
|
Agitation |
7.6 |
10.9 |
12.5 |
16.7 |
|
Anxiety |
1.5 |
7.8 |
4.7 |
1.5 |
|
Nervousness |
1.5 |
6.3 |
1.6 |
0 |
|
Somnolence |
0 |
3.1 |
9.4a |
4.5 |
|
Extrapyramidal side effects |
10.6 |
10.9 |
25.0a |
25.8a |
|
Headache |
4.5 |
15.6a |
9.4 |
7.6 |
|
Dizziness |
0 |
9.4a |
10.9b |
0 |
|
Dyspepsia |
4.5 |
9.4 |
6.3 |
4.5 |
|
Vomiting |
1.5 |
6.3 |
6.3 |
3.0 |
|
Nausea |
0 |
6.3 |
3.1 |
1.5 |
|
Constipation |
0 |
1.6 |
6.3 |
1.5 |
|
Rhinitis |
6.1 |
15.6 |
6.3 |
4.5 |
|
Coughing |
1.5 |
9.4 |
3.1 |
3.0 |
|
Sinusitis |
1.5 |
6.3 |
1.6 |
0 |
|
Fever |
0 |
6.3 |
3.1 |
1.5 |
|
Tachycardia |
0 |
4.7 |
6.3 |
1.5 |
|
aP<0.05 versus placebo. bP<0.01 versus placebo. Source. Adapted from Marder and Meibach 1994. |
|||||
Metabolic Effects
Weight gain with risperidone is intermediate—that is, the degree of weight gain is between that associated with agents like molindone, amisulpride, and ziprasidone, which appear to be relatively weight neutral, and that associated with agents like clozapine, olanzapine, and low-potency phenothiazines (Rummel-Kluge et al. 2010; Sikich et al. 2008). In a meta-analysis of controlled trials, Allison et al. (1999), using a random effects model, estimated the mean weight gain at 10 weeks with risperidone to be 2.0 kg, compared with 0.5 kg with haloperidol, 3.5 kg with olanzapine, and 4.0 kg with clozapine. In the CATIE, in which risperidone had the lowest rate of discontinuation due to side effects, risperidone treatment was associated with a mean monthly weight gain of 0.4 lb, olanzapine with 2.0 lbs, and quetiapine with 0.5 lb; by contrast, perphenazine and ziprasidone were associated with a mean monthly weight loss of 0.2 lb and 0.3 lb, respectively (Lieberman et al. 2005). Although determining the risk for hyperglycemia is complex, and results of studies have not been completely consistent, it appears that risperidone does not produce insulin resistance to the degree associated with olanzapine and clozapine (American Diabetes Association et al. 2004; Henderson et al. 2006; Lieberman et al. 2005). A meta-analysis of head-to-head comparisons of SGAs found that risperidone produced more cholesterol elevation than aripiprazole and ziprasidone and less elevation than olanzapine and quetiapine (Rummel-Kluge et al. 2010). Metabolic side effects in children tend to be more severe than those in adults; for example, in one study of pediatric patients younger than 20 years, risperidone produced a mean weight gain of 5.3 kg (11.7 lb) over the 12-week treatment period (Correll et al. 2009). Metabolic side effects with paliperidone appear to be similar to those with risperidone.
Extrapyramidal Side Effects
Significant reductions in EPS with risperidone compared with high-dose haloperidol were a consistent finding in the North American trials (Chouinard et al. 1993; Marder and Meibach 1994). Measurement of EPS in the placebo group was complicated because 25% of the subjects were receiving depot antipsychotics prior to enrollment. Risperidone produced significantly fewer parkinsonian side effects than did haloperidol (20 mg/day), based on several measures, including self-report, change scores on the Extrapyramidal Symptom Rating Scale (ESRS), and use of anticholinergic medication. Patients receiving risperidone (2 mg/day and 6 mg/day) did not differ from the group receiving placebo in mean ratings of parkinsonism and in the use of anticholinergic medication. Parkinsonism change scores were significantly correlated with the risperidone dosage (r=0.94); however, risperidone (16 mg/day) was associated with fewer parkinsonian side effects than was haloperidol. Dystonia occurred in six of the patients treated with risperidone (1.7%) versus two of the patients treated with haloperidol (2.4%). Dystonia rates did not differ between treatment groups, and the rates did not exhibit a relationship to risperidone dosage.
In the large European multicenter trial, maximum ratings of parkinsonism, hyperkinesis, and dystonia were greater with haloperidol (10 mg/day) than with all dosages of risperidone (maximum of 12 mg/day), and anticholinergic dosing was accordingly higher in the group treated with haloperidol (Peuskens 1995). Similarly, in a flexible-dose comparison of risperidone (mean dosage 4.9 mg/day) and haloperidol (mean dosage 11.7 mg/day) for prevention of relapse, EPS rates and use of anticholinergic medication significantly favored the group taking risperidone (Csernansky et al. 2002). However, in a smaller double-blind, flexible-dose trial comparing risperidone (5–15 mg/day) and the moderate-potency FGA agent perphenazine (16–48 mg/day) in 107 patients, no difference in EPS rates was observed (Høyberg et al. 1993), indicating that the potency of the comparator agent may in part determine the relative benefit of risperidone for EPS. Of interest, in a study of low-dose risperidone (mean dosage 1.2 mg/day) in children with behavioral disorders, ratings of EPS did not differ between risperidone and placebo (Aman et al. 2002). No differences in EPS ratings were found among any treatment groups in the CATIE (Lieberman et al. 2005), although discontinuation rates due to EPS significantly differed, with perphenazine producing the highest discontinuation rate (8%) and olanzapine (2%), risperidone (3%), and quetiapine (3%) producing the lowest.
The experience with tardive dyskinesia (TD) in patients treated with risperidone has been promising. Jeste et al. (1999) randomly treated 122 elderly patients with low-dosage haloperidol (median daily dose 1 mg) versus risperidone (median daily dose 1 mg). The very high rates of treatment-emergent TD typically found in geriatric patients make this sample a sensitive assay for TD risk. After 9 months, treatment-emergent TD rates were 30% with haloperidol versus less than 5% with risperidone. Risperidone was also noted to decrease dyskinetic movements compared with haloperidol in a Canadian multicenter trial reported by Chouinard et al. (1993), and it was associated with a treatment-emergent TD rate of 0.6%, compared with a rate of 2.7% with haloperidol, in a relapse prevention trial reported by Csernansky et al. (2002).
Hyperprolactinemia
Unlike other SGA agents, risperidone and paliperidone substantially increase serum prolactin levels—in some studies, to a greater degree than does haloperidol (Kearns et al. 2000; Markianos et al. 1999)—although prolactin levels may decrease over time (Eberhard et al. 2007; Findling et al. 2003). The relationship between serum prolactin concentrations and clinical side effects remains somewhat unclear, however. Kleinberg et al. (1999) analyzed combined results from the North American and European multicenter registration trials, which included plasma prolactin concentrations from 841 patients and clinical ratings of symptoms associated with hyperprolactinemia from 1,884 patients. Mean prolactin levels significantly correlated with risperidone dosage; risperidone 6 mg/day produced elevations roughly comparable to those seen with haloperidol 20 mg/day and significantly higher than those seen with haloperidol 10 mg/day. The combined incidence of amenorrhea and galactorrhea in women, which varied between 8% and 12%, was similar for all dosages of risperidone and haloperidol (10 mg/day). Because symptom frequencies were available only for 14 women treated with placebo, comparisons with placebo were not informative. Sexual dysfunction or gynecomastia occurred in 15% of men treated with risperidone (4–6 mg/day), compared with 14% of men treated with haloperidol (10 mg/day) and 8% of men in the placebo group. Compared with placebo, ejaculatory dysfunction was significantly more frequent only in the group treated with risperidone (12–16 mg/day). Mean plasma prolactin levels were not significantly related to clinical side effects for either men or women. Decreased libido also did not differ between treatment groups and did not correlate with plasma prolactin levels. In the CATIE, prolactin levels increased by a mean of 15.4 ng/mL with risperidone, compared with a 0.4-ng/mL mean elevation with perphenazine and decreases of 4.5–9.3 ng/mL with the other SGAs (Lieberman et al. 2005). Despite having significantly higher serum prolactin concentrations, patients treated with risperidone did not report significantly higher rates of sexual dysfunction, gynecomastia, galactorrhea, or irregular menses.
Two reports of clinical trials with extended-release paliperidone have indicated low levels of prolactin-related side effects (1% and 4%) (Kane et al. 2007; Kramer et al. 2007). However, in the one publication that reported prolactin levels, substantial increases in mean plasma prolactin concentrations were observed (males: 17.4 ng/mL at baseline to 45.3 ng/mL at week 6; females: 38.0 ng/mL to 124.5 ng/mL) (Kane et al. 2007). A 13-week comparison of paliperidone palmitate with risperidone microspheres reported that paliperidone palmitate was associated with moderately higher elevations from baseline in prolactin levels compared with risperidone (women: 21.8 vs. 15.6 ng/mL; men: 9.4 vs. 6.0 ng/mL) (Pandina et al. 2011); however, another study found that the proportion of patients with abnormally elevated prolactin levels was higher in the group receiving LAI risperidone than in that receiving paliperidone palmitate (Fleischhacker et al. 2012). Two preliminary studies with risperidone found that plasma prolactin concentrations correlated with 9-hydroxyrisperidone (paliperidone) concentrations and not with risperidone concentrations (Melkersson 2006; Troost et al. 2007). The ratio of 9-hydroxyrisperidone levels to risperidone levels also correlated with prolactin concentration (Troost et al. 2007); in agreement with this finding, rapid metabolizers of CYP2D6 were found to have higher prolactin concentrations than poor metabolizers (Troost et al. 2007).
Cardiovascular Effects
Because of relatively high affinities for adrenoreceptors, risperidone would be expected to produce orthostatic hypotension. However, by following a 3- to 7-day dosage escalation schedule, initial postural hypotension and tachycardia have been avoided in clinical trials, with only rare cases of hypotension and syncope reported (Chouinard et al. 1993; Marder and Meibach 1994). Risperidone has very modest effects on cardiac conduction. No significant prolongation of the QTc interval was detected at dosages of up to 25 mg/day in early safety trials, and no relationship between QTc interval and risperidone dose was apparent (Mesotten et al. 1989). In the CATIE, risperidone was associated with the least QTc prolongation (mean 0.2 msec) and quetiapine with the most (mean 5.9 msec), although differences were not statistically significant (Lieberman et al. 2005). A mean QTc prolongation of 10 msec, measured after peak absorption of risperidone (16 mg/day), was found in a study comparing SGAs and FGAs, according to data filed with the FDA by Pfizer Inc. (Harrigan et al. 2004). In a retrospective cohort study of Medicaid enrollees in Tennessee, risperidone was associated with a 2.9-fold increase in rate of sudden cardiac death, compared with a 1.61-fold increase with haloperidol and a 3.67-fold increase with clozapine (Ray et al. 2009).
High Dose and Overdose
Mesotten et al. (1989) reported the results of a safety trial involving 17 inpatients with psychosis in which, following a washout of previous medication, risperidone was started at 10 mg/day, and the dosage was then increased weekly by 5 mg/day to a maximum of 25 mg/day. Despite extremely high doses, sedation was the only prominent side effect. Although risperidone does not bind significantly to muscarinic cholinergic receptors, transient dry mouth, blurred vision, and urinary retention were observed in individual subjects. Palpitations occurred in two subjects. Heart rate significantly increased during the trial, and blood pressure slightly decreased; however; no cases of significant hypotension were reported. An endocrine battery, including plasma triiodothyronine, thyroid-stimulating hormone, growth hormone, prolactin, follicle-stimulating hormone, luteinizing hormone, and cortisol levels, was performed, and only prolactin was found to be affected. Reported overdoses with risperidone have generally been benign, with moderate QT prolongation and no serious cardiac complications (Brown et al. 1993; Lo Vecchio et al. 1996).
Drug–Drug Interactions
Because CYP2D6 status affects the half-life of risperidone and the relative ratio of risperidone to 9-hydroxyrisperidone in plasma, the total serum concentration of the “active moiety,” or the sum of the concentrations of risperidone and 9-hydroxyrisperidone, may be significantly increased with addition of a CYP2D6 inhibitor (e.g., fluoxetine) in rapid metabolizers but not in poor metabolizers (Bondolfi et al. 2002; Spina et al. 2002). In one study of 9 patients treated with risperidone, addition of fluoxetine resulted in a 75% increase in blood levels of the active moiety (risperidone+9-hydroxyrisperidone); two patients developed parkinsonian side effects (Spina et al. 2002). Paliperidone plasma concentrations are not influenced by CYP2D6 status, nor are paliperidone plasma concentrations likely to be affected by drug–drug interactions. It has been hypothesized that the addition of a CYP2D6 inhibitor (e.g., fluoxetine) could decrease risperidone-induced prolactin elevation by increasing the ratio of risperidone to 9-hydroxyrisperidone (Troost et al. 2007), although this has not been rigorously tested.
Conclusion
Risperidone was the first antipsychotic agent developed specifically to exploit the clinical advantages of combined D2 and 5-HTA2 receptor antagonism. α-Adrenergic antagonism additionally may contribute to the antipsychotic and cognition-enhancing effects of risperidone. Risperidone’s active metabolite, paliperidone, is pharmacologically quite similar to the parent drug and comprises roughly 80%–90% of the serum concentration of the active moiety (risperidone + paliperidone) in most patients treated with risperidone. Risperidone and paliperidone are generally quite well tolerated, producing moderate weight gain and mild sedation. Initial dosage titration is necessary to prevent orthostatic blood pressure changes and dizziness, although this may be less necessary with extended-release paliperidone. EPS are dose related and are typically less common with risperidone than with haloperidol but more common with risperidone compared with other SGAs. Risperidone and paliperidone markedly elevate prolactin levels, although the relationship between plasma prolactin concentrations and clinical symptoms is complex. The efficacy of risperidone was initially established in comparison with high-dose haloperidol, against which it was significantly more effective for all five symptom clusters derived from the PANSS. However, the magnitude of difference in effect size was not large for individual symptom clusters. In the CATIE, risperidone (at a mean daily dosage of 3.9 mg) did not differ from perphenazine in rates of discontinuation due to lack of effectiveness but was less effective than olanzapine (Lieberman et al. 2005). The risperidone microspheres product was the first FDA-approved SGA long-acting IM formulation, and paliperidone palmitate was the first SGA depot formulation that could be administered at an interval of every 4 weeks. PP3M is the first depot antipsychotic that can be administered on a quarterly schedule. Overall, risperidone and paliperidone are well-tolerated SGA agents with efficacy comparable to that of most other agents in their class.
References
Allison DB, Mentore JL, Heo M, et al: Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 156(11):1686–1696, 1999 10553730
Aman MG, De Smedt G, Derivan A, et al; Risperidone Disruptive Behavior Study Group: Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry 159(8):1337–1346, 2002 12153826
American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity: Consensus development conference on antipsychotic drugs and diabetes and obesity. Diabetes Care 27(2):596–601, 2004 14747245
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition. Washington, DC, American Psychiatric Association, 1994
Anderson CB, True JE, Ereshefsky L, et al: Risperidone dose, plasma levels, and response. Presentation at the 146th annual meeting of the American Psychiatric Association, San Francisco, CA, May 22–27, 1993
Ansoms C, Backer-Dierick GD, Vereecken JL: Sleep disorders in patients with severe mental depression: double-blind placebo-controlled evaluation of the value of pipamperone (Dipiperon). Acta Psychiatr Scand 55(2):116–122, 1977 320830
Arakawa R, Ito H, Takano A, et al: Dose-finding study of paliperidone ER based on striatal and extrastriatal dopamine D2 receptor occupancy in patients with schizophrenia. Psychopharmacology (Berl) 197(2):229–235, 2008 18058087
Attard A, Olofinjana O, Cornelius V, et al: Paliperidone palmitate long-acting injection-prospective year-long follow-up of use in clinical practice. Acta Psychiatr Scand 130(1):46–51, 2014 24117209
Berwaerts J, Liu Y, Gopal S, et al: Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry 72(8):830–839, 2015 25820612
Bai YM, Ting Chen T, Chen JY, et al: Equivalent switching dose from oral risperidone to risperidone long-acting injection: a 48-week randomized, prospective, single-blind pharmacokinetic study. J Clin Psychiatry 68(8):1218–1225, 2007 17854246
Bersani G, Grispini A, Marini S, et al: 5-HT2 antagonist ritanserin in neuroleptic-induced parkinsonism: a double-blind comparison with orphenadrine and placebo. Clin Neuropharmacol 13(6):500–506, 1990 2125857
Bondolfi G, Eap CB, Bertschy G, et al: The effect of fluoxetine on the pharmacokinetics and safety of risperidone in psychotic patients. Pharmacopsychiatry 35(2):50–56, 2002 11985287
Borison RL, Diamond B, Pathiraja A, Meibach RC: Pharmacokinetics of risperidone in chronic schizophrenic patients. Psychopharmacol Bull 30(2):193–197, 1994 7530379
Brown K, Levy H, Brenner C, et al: Overdose of risperidone. Ann Emerg Med 22(12): 1908–1910, 1993 7694530
Busatto GF, Kerwin RW: Perspectives on the role of serotonergic mechanisms in the pharmacology of schizophrenia. J Psychopharmacol 11(1):3–12, 1997 9097883
Ceulemans DL, Gelders YG, Hoppenbrouwers ML, et al: Effect of serotonin antagonism in schizophrenia: a pilot study with setoperone. Psychopharmacology (Berl) 85(3):329–332, 1985 3923519
Chouinard G, Jones B, Remington G, et al: A Canadian multicenter placebo-controlled study of fixed doses of risperidone and haloperidol in the treatment of chronic schizophrenic patients. J Clin Psychopharmacol 13(1):25–40, 1993 7683702
Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd Edition. Hillsdale, NJ, Lawrence Erlbaum, 1988
Colpaert FC, Meert TF, Niemegeers CJ, Janssen PA: Behavioral and 5-HT antagonist effects of ritanserin: a pure and selective antagonist of LSD discrimination in rat. Psychopharmacology (Berl) 86(1–2):45–54, 1985 2862659
Correll CU, Manu P, Olshanskiy V, et al: Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents (erratum in JAMA 302:2322, 2009). JAMA 302(16):1765–1773, 2009 19861668
Coull JT, Sahakian BJ, Hodges JR: The alpha(2) antagonist idazoxan remediates certain attentional and executive dysfunction in patients with dementia of frontal type. Psychopharmacology (Berl) 123(3):239–249, 1996 8833417
Csernansky JG, Mahmoud R, Brenner R; Risperidone-USA-79 Study Group: A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 346(1):16–22, 2002 11777998
Cuesta MJ, Jalón EG, Campos MS, Peralta V: Cognitive effectiveness of olanzapine and risperidone in first-episode psychosis. Br J Psychiatry 194(5):439–445, 2009 19407274
Davis JM, Chen N, Glick ID: A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry 60(6): 553–564, 2003 12796218
de Leon J, Wynn G, Sandson NB: The pharmacokinetics of paliperidone versus risperidone. Psychosomatics 51(1):80–88, 2010 20118446
Duinkerke SJ, Botter PA, Jansen AA, et al: Ritanserin, a selective 5-HT2/1C antagonist, and negative symptoms in schizophrenia. A placebo-controlled double-blind trial. Br J Psychiatry 163:451–455, 1993 7902766
Eberhard J, Lindström E, Holstad M, Levander S: Prolactin level during 5 years of risperidone treatment in patients with psychotic disorders. Acta Psychiatr Scand 115(4): 268–276, 2007 17355517
Findling RL, Kusumakar V, Daneman D, et al: Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry 64(11):1362–1369, 2003 14658952
Fleischhacker WW, Gopal S, Lane R, et al: A randomized trial of paliperidone palmitate and risperidone long-acting injectable in schizophrenia. Int J Neuropsychopharmacol 15(1):107–118, 2012 21777507
Gelders YG: Thymosthenic agents, a novel approach in the treatment of schizophrenia. Br J Psychiatry Suppl 155(5):33–36, 1989 2481482
Gellman RL, Aghajanian GK: Serotonin2 receptor-mediated excitation of interneurons in piriform cortex: antagonism by atypical antipsychotic drugs. Neuroscience 58(3):515–525, 1994 7513386
Geyer MA, Swerdlow NR, Mansbach RS, Braff DL: Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull 25(3):485–498, 1990 2292046
Goff DC, Coyle JT: The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 158(9):1367–1377, 2001 11532718
Goff DC, Evins AE: Negative symptoms in schizophrenia: neurobiological models and treatment response. Harv Rev Psychiatry 6(2):59–77, 1998 10370450
Green MF, Marshall BD Jr, Wirshing WC, et al: Does risperidone improve verbal working memory in treatment-resistant schizophrenia? Am J Psychiatry 154(6):799–804, 1997 9167507
Green MF, Marder SR, Glynn SM, et al: The neurocognitive effects of low-dose haloperidol: a two-year comparison with risperidone. Biol Psychiatry 51(12):972–978, 2002 12062881
Haapalinna A, Sirviö J, MacDonald E, et al: The effects of a specific alpha(2)-adrenoceptor antagonist, atipamezole, on cognitive performance and brain neurochemistry in aged Fisher 344 rats. Eur J Pharmacol 387(2):141–150, 2000 10650154
Harrigan EP, Miceli JJ, Anziano R, et al: A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol 24(1):62–69, 2004 14709949
Harris MS, Wiseman CL, Reilly JL, et al: Effects of risperidone on procedural learning in antipsychotic-naive first-episode schizophrenia. Neuropsychopharmacology 34(2):468–476, 2009 18536701
Harvey PD, Rabinowitz J, Eerdekens M, Davidson M: Treatment of cognitive impairment in early psychosis: a comparison of risperidone and haloperidol in a large long-term trial. Am J Psychiatry 162(10):1888–1895, 2005 16199835
Henderson DC, Copeland PM, Borba CP, et al: Glucose metabolism in patients with schizophrenia treated with olanzapine or quetiapine: a frequently sampled intravenous glucose tolerance test and minimal model analysis. J Clin Psychiatry 67(5):789–797, 2006 16841629
Hertel P, Fagerquist MV, Svensson TH: Enhanced cortical dopamine output and antipsychotic-like effects of raclopride by alpha2 adrenoceptor blockade. Science 286(5437):105–107, 1999 10506554
Heykants J, Huang ML, Mannens G, et al: The pharmacokinetics of risperidone in humans: a summary. J Clin Psychiatry 55 (suppl):13–17, 1994 7520903
Høyberg OJ, Fensbo C, Remvig J, et al: Risperidone versus perphenazine in the treatment of chronic schizophrenic patients with acute exacerbations. Acta Psychiatr Scand 88(6):395–402, 1993 7508675
Hough D, Gopal S, Vijapurkar U, et al: Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res 116(2–3):107–117, 2010 19959339
Janssen PA, Niemegeers CJ, Awouters F, et al: Pharmacology of risperidone (R 64 766), a new antipsychotic with serotonin-S2 and dopamine-D2 antagonistic properties. J Pharmacol Exp Ther 244(2): 685–693, 1988 2450200
Jeste DV, Lacro JP, Bailey A, et al: Lower incidence of tardive dyskinesia with risperidone compared with haloperidol in older patients. J Am Geriatr Soc 47(6): 716–719, 1999 10366172
Kane JM, Eerdekens M, Lindenmayer JP, et al: Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry 160(6):1125–1132, 2003 12777271
Kane J, Canas F, Kramer M, et al: Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr Res 90(1–3):147–161, 2007 17092691
Kapur S, Seeman P: Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics? a new hypothesis. Am J Psychiatry 158(3): 360–369, 2001 11229973
Kapur S, Zipursky RB, Remington G: Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry 156(2):286–293, 1999 9989565
Kearns AE, Goff DC, Hayden DL, Daniels GH: Risperidone-associated hyperprolactinemia. Endocr Pract 6(6):425–429, 2000 11155212
Keefe RS, Young CA, Rock SL, et al; HGGN Study Group: One-year double-blind study of the neurocognitive efficacy of olanzapine, risperidone, and haloperidol in schizophrenia. Schizophr Res 81(1):1–15, 2006 16202565
Keefe RS, Bilder RM, Davis SM, et al; CATIE Investigators; Neurocognitive Working Group: Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry 64(6):633–647, 2007 17548746
Keks NA, Ingham M, Khan A, Karcher K: Long-acting injectable risperidone v. olanzapine tablets for schizophrenia or schizoaffective disorder. Randomised, controlled, open-label study. Br J Psychiatry 191:131–139, 2007 17666497
Kleinberg DL, Davis JM, de Coster R, et al: Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol 19(1):57–61, 1999 9934944
Kramer M, Simpson G, Maciulis V, et al: Paliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 27(1):6–14, 2007 17224706
Kramer M, Litman R, Hough D, et al: Paliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety study. Int J Neuropsychopharmacol 13(5):635–647, 2010 19941696
Krystal JH, Karper LP, Seibyl JP, et al: Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51(3):199–214, 1994 8122957
Leucht S, Corves C, Arbter D, et al: Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373(9657):31–41, 2009 19058842
Leucht S, Cipriani A, Spineli L, et al: Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382(9896):951–962, 2013 23810019
Leysen JE, Niemegeers CJE, Tollenaere JP, Laduron PM: Serotonergic component of neuroleptic receptors. Nature 272(5649): 168–171, 1978 564466
Leysen JE, Gommeren W, Mertens J, et al: Comparison of in vitro binding properties of a series of dopamine antagonists and agonists for cloned human dopamine D2S and D2L receptors and for D2 receptors in rat striatal and mesolimbic tissues, using [125I] 2′-iodospiperone. Psychopharmacology (Berl) 110(1–2):27–36, 1993a 7870895
Leysen JE, Janssen PM, Schotte A, et al: Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl) 112 (1 suppl):S40–S54, 1993b 7530377
Leysen JE, Janssen PM, Megens AA, Schotte A: Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry 55 (suppl):5–12, 1994 7520908
Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353(12):1209–1223, 2005 16172203
Litman RE, Su TP, Potter WZ, et al: Idazozan and response to typical neuroleptics in treatment-resistant schizophrenia. Comparison with the atypical neuroleptic, clozapine. Br J Psychiatry 168(5):571–579, 1996 8733795
Lo Vecchio F, Hamilton RJ, Hoffman RJ: Risperidone overdose (letter). Am J Emerg Med 14(1):95–96, 1996 8630169
Mahmoud RA, Pandina GJ, Turkoz I, et al: Risperidone for treatment-refractory major depressive disorder: a randomized trial. Ann Intern Med 147(9):593–602, 2007 17975181
Malla A, Chue P, Jordan G, et al: An exploratory open-label randomized trial comparing risperidone long acting injectable (RLAI) with oral antipsychotic medication in the treatment of early psychosis. Clin Schizophr Relat Psychoses 17:1–26, 2013 23773886
Mannens G, Huang M-L, Meuldermans W, et al: Absorption, metabolism, and excretion of risperidone in humans. Drug Metab Dispos 21(6):1134–1141, 1993 7507814
Marder SR, Meibach RC: Risperidone in the treatment of schizophrenia. Am J Psychiatry 151(6):825–835, 1994 7514366
Marder SR, Davis JM, Chouinard G: The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry 58(12): 538–546, 1997 9448657
Markianos M, Hatzimanolis J, Lykouras L: Gonadal axis hormones in male schizophrenic patients during treatment with haloperidol and after switch to risperidone. Psychopharmacology (Berl) 143(3): 270–272, 1999 10353429
McCracken JT, McGough J, Shah B, et al; Research Units on Pediatric Psychopharmacology Autism Network: Risperidone in children with autism and serious behavioral problems. N Engl J Med 347(5):314–321, 2002 12151468
McDougle CJ, Holmes JP, Carlson DC, et al: A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry 55(7):633–641, 1998 9672054
McDougle CJ, Epperson CN, Pelton GH, et al: A double-blind, placebo-controlled study of risperidone addition in serotonin reuptake inhibitor-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 57(8):794–801, 2000 10920469
McDougle CJ, Scahill L, Aman MG, et al: Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 162(6):1142–1148, 2005 15930063
McEvoy JP, Byerly M, Hamer RM, et al: Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA 311(19):1978–1987, 2014 24846035
Meert TF, Colpaert FC: Effects of S2-antagonists in two conflict procedures that involve exploratory behavior. Psychopharmacology (Berl) 88(4):445–450, 1986 2871580
Meert TF, de Haes P, Janssen PA: Risperidone (R 64 766), a potent and complete LSD antagonist in drug discrimination by rats. Psychopharmacology (Berl) 97(2):206–212, 1989 2471220
Megens AA, Awouters FHL, Niemegeers CJE: Differential effects of the new antipsychotic risperidone on large and small motor movements in rats: a comparison with haloperidol. Psychopharmacology (Berl) 95(4):493–496, 1988 2463650
Melkersson KI: Prolactin elevation of the antipsychotic risperidone is predominantly related to its 9-hydroxy metabolite. Hum Psychopharmacol 21(8):529–532, 2006 17094165
Meltzer HY, Bastani B, Ramirez L, Matsubara S: Clozapine: new research on efficacy and mechanism of action. Eur Arch Psychiatry Neurol Sci 238(5–6):332–339, 1989 2569975
Meltzer HY, Bobo WV, Nuamah IF, et al: Efficacy and tolerability of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 6-week, placebo-controlled studies. J Clin Psychiatry 69(5):817–829, 2008 18466043
Mesotten F, Suy E, Pietquin M, et al: Therapeutic effect and safety of increasing doses of risperidone (R 64766) in psychotic patients. Psychopharmacology (Berl) 99(4):445–449, 1989 2480616
Nasrallah HA, Gopal S, Gassmann-Mayer C, et al: A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology 35(10):2072–2082, 2010 20555312
Nussbaum AM, Stroup TS: Paliperidone for treatment of schizophrenia. Schizophr Bull 34(3):419–422, 2008 18375569
Nyberg S, Eriksson B, Oxenstierna G, et al: Suggested minimal effective dose of risperidone based on PET-measured D2 and 5-HT2A receptor occupancy in schizophrenic patients. Am J Psychiatry 156(6):869–875, 1999 10360125
Pandina GJ, Canuso CM, Turkoz I, et al: Adjunctive risperidone in the treatment of generalized anxiety disorder: a double-blind, prospective, placebo-controlled, randomized trial. Psychopharmacol Bull 40(3):41–57, 2007 18007568
Pandina G, Lane R, Gopal S, et al: A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 35(1): 218–226, 2011 21092748
Peuskens J; Risperidone Study Group: Risperidone in the treatment of patients with chronic schizophrenia: a multi-national, multi-centre, double-blind, parallel-group study versus haloperidol. Br J Psychiatry 166(6):712–726, discussion 727–733, 1995 7545060
Poyurovsky M, Shardorodsky M, Fuchs C, et al: Treatment of neuroleptic-induced akathisia with the 5-HT2 antagonist mianserin. Double-blind, placebo-controlled study. Br J Psychiatry 174:238–242, 1999 10448449
Quiroz JA, Yatham LN, Palumbo JM, et al: Risperidone long-acting injectable monotherapy in the maintenance treatment of bipolar I disorder. Biol Psychiatry 68(2):156–162, 2010 20227682
Ray WA, Chung CP, Murray KT, et al: Atypical antipsychotic drugs and the risk of sudden cardiac death (erratum in N Engl J Med 361:1814, 2009). N Engl J Med 360(3):225–235, 2009 19144938
Reilly JL, Harris MS, Keshavan MS, Sweeney JA: Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch Gen Psychiatry 63(11):1189–1197, 2006 17088499
Remington G, Mamo D, Labelle A, et al: A PET study evaluating dopamine D2 receptor occupancy for long-acting injectable risperidone. Am J Psychiatry 163(3): 396–401, 2006 16513859
Rendell JM, Gijsman HJ, Bauer MS, et al: Risperidone alone or in combination for acute mania. Cochrane Database Syst Rev (1):CD004043, 2006 16437472
Research Units on Pediatric Psychopharmacology Autism Network: Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry 162(7):1361–1369, 2005 15994720
Reyntjens A, Gelders YG, Hoppenbrouwers M, et al: Thymostenic effects of ritanserin (R55 667), a centrally active serotonin-S2 receptor blocker. Drug Dev Res 8(1–4):205–211, 1986
Richelson E: Preclinical pharmacology of neuroleptics: focus on new generation compounds. J Clin Psychiatry 57 (suppl 11):4–11, 1996 8941166
Robinson DG, Woerner MG, Napolitano B, et al: Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. Am J Psychiatry 163(12):2096–2102, 2006 17151160
Roose K, Gelders Y, Heylen S: Risperidone (R64 766) in psychotic patients. A first clinical therapeutic exploration. Acta Psychiatr Belg 88(3):233–241, 1988 2466393
Rosenheck RA, Krystal JH, Lew R, et al; Long-acting risperidone and oral antipsychotics in unstable schizophrenia. N Engl J Med 364(9):842–851, 2011 21366475
Rummel-Kluge C, Komossa K, Schwarz S, et al: Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 123(2–3):225–233, 2010 20692814
Saller CF, Czupryna MJ, Salama AI: 5-HT2 receptor blockade by ICI 169,369 and other 5-HT2 antagonists modulates the effects of D-2 dopamine receptor blockade. J Pharmacol Exp Ther 253(3):1162–1170, 1990 2141636
Savitz AJ, Xu H, Gopal S, et al: Efficacy and safety of paliperidone palmitate 3-month formulation for patients with schizophrenia: a randomized, multicenter, double-blind, noninferiority study. Int J Neuropsychopharmacol 19(7):1–14, 2016 26902950
Schmidt CJ, Sorensen SM, Kehne JH, et al: The role of 5-HT2A receptors in antipsychotic activity. Life Sci 56(25):2209–2222, 1995 7791509
Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group: Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med 355(15):1525–1538, 2006 17035647
Schotte A, Janssen PF, Gommeren W, et al: Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 124(1–2):57–73, 1996 8935801
Seeman P: Atypical antipsychotics: mechanism of action. Can J Psychiatry 47(1):27–38, 2002 11873706
Sikich L, Frazier JA, McClellan J, et al: Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study (erratum in Am J Psychiatry 165:1495, 2008). Am J Psychiatry 165(11):1420–1431, 2008 18794207
Simpson GM, Mahmoud RA, Lasser RA, et al: A 1-year double-blind study of 2 doses of long-acting risperidone in stable patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry 67(8):1194–1203, 2006 16965196
Spina E, Avenoso A, Scordo MG, et al: Inhibition of risperidone metabolism by fluoxetine in patients with schizophrenia: a clinically relevant pharmacokinetic drug interaction. J Clin Psychopharmacol 22(4):419–423, 2002 12172343
Stathis P, Antoniou K, Papadopoulou-Daifotis Z, et al: Risperidone: a novel antipsychotic with many “atypical” properties? Psychopharmacology (Berl) 127(3):181–186, 1996 8912395
Stroup TS, McEvoy JP, Swartz MS, et al: The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull 29(1):15–31, 2003 12908658
Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators: Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 163(4):611–622, 2006 16585435
Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators: Effectiveness of olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia after discontinuing perphenazine: a CATIE study. Am J Psychiatry 164(3):415–427, 2007 17329466
Subotnik KL, Casaus LR, Ventura J, et al: Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry 72(8):822–829, 2015 26107752
Svensson TH, Tung C-S, Grenhoff J: The 5-HT2 antagonist ritanserin blocks the effect of pre-frontal cortex inactivation on rat A10 dopamine neurons in vivo. Acta Physiol Scand 136(3):497–498, 1989 2568733
Svensson TH, Mathé JM, Andersson JL, et al: Mode of action of atypical neuroleptics in relation to the phencyclidine model of schizophrenia: role of 5-HT2 receptor and alpha 1-adrenoceptor antagonism [corrected]. J Clin Psychopharmacol 15 (1 suppl 1):11S–18S, 1995 7730496
Takahashi N, Takahashi M, Saito T, et al: Randomized, placebo-controlled, double-blind study assessing the efficacy and safety of paliperidone palmitate in Asian patients with schizophrenia. Neuropsychiatr Dis Treat 8:1889–1898, 2013 24353421
Tiihonen J, Haukka J, Taylor M, et al: A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia (erratum in Am J Psychiatry 169:223, 2012). Am J Psychiatry 168(6):603–609, 2011 21362741
Troost PW, Lahuis BE, Hermans MH, et al: Prolactin release in children treated with risperidone: impact and role of CYP2D6 metabolism. J Clin Psychopharmacol 27(1):52–57, 2007 17224713
Ugedo L, Grenhoff J, Svensson TH: Ritanserin, a 5-HT2 receptor antagonist, activates midbrain dopamine neurons by blocking serotonergic inhibition. Psychopharmacology (Berl) 98(1):45–50, 1989 2524859
Varty GB, Bakshi VP, Geyer MA: M100907, a serotonin 5-HT2A receptor antagonist and putative antipsychotic, blocks dizocilpine-induced prepulse inhibition deficits in Sprague-Dawley and Wistar rats. Neuropsychopharmacology 20(4):311–321, 1999 10088132
Volavka J, Czobor P, Sheitman B, et al: Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry 159(2): 255–262, 2002 11823268
Wadenberg ML, Hicks PB, Richter JT, Young KA: Enhancement of antipsychoticlike properties of raclopride in rats using the selective serotonin2A receptor antagonist MDL 100,907. Biol Psychiatry 44(6): 508–515, 1998 9777184
Wirshing DA, Marshall BD Jr, Green MF, et al: Risperidone in treatment-refractory schizophrenia. Am J Psychiatry 156(9): 1374–1379, 1999 10484947