CHAPTER 29
Aripiprazole and Brexpiprazole
Rolando Gonzalez, M.D.
Martin T. Strassnig, M.D.
History and Discovery
In this chapter, we review the preclinical and clinical pharmacology of aripiprazole and brexpiprazole, two novel atypical (second-generation) antipsychotics. Aripiprazole is a dihydroquinolinone antipsychotic agent. Chemically, it is unrelated to phenothiazines, butyrophenones, or thienobenzodiazepines. Pharmacologically, it exhibits a novel mechanism of action, combining partial agonist activity at dopamine type 2 (D2), dopamine type 3 (D3), and serotonin type 1A (5-HT1A) receptors with antagonist activity at serotonin type 2A (5-HT2A) and D2 receptors (Burris et al. 2002; Jordan et al. 2002). Aripiprazole represents a significant innovation, following the introduction of typical (first-generation) and atypical antipsychotics, in the pharmacology of therapeutic agents for psychotic disorders.
Brexpiprazole has been developed with a goal for further stabilization of dopaminergic transmission, more specifically regarding an optimal level of D2 intrinsic activity. The pharmacological profile of brexpiprazole is similar to that of aripiprazole, including combined partial agonist activity at D2, D3, and 5-HT1A receptors with antagonist activity at 5-HT2A and D2 receptors. However, brexpiprazole has lower intrinsic activity at D2 receptors, high-potency partial agonism at 5-HT1A receptors, and stronger antagonism at 5-HT2A receptors (Maeda et al. 2014b).
Although effective in alleviating psychotic symptoms and preventing their recurrence, the typical agents are ineffective in up to 40% of patients with schizophrenia, lack efficacy against the negative symptoms and cognitive deficits of schizophrenia, and are associated with a considerable burden of extrapyramidal side effects (EPS).
The atypical antipsychotics are partially effective against negative as well as positive symptoms and are associated with fewer EPS compared with the conventional antipsychotics. Nevertheless, individual atypical agents are associated with side effects such as weight gain and other metabolic abnormalities, hyperprolactinemia, QTc prolongation, and alterations in glucose and lipid levels (Allison et al. 1999; Glassman and Bigger 2001; Koro et al. 2002a, 2002b; McIntyre et al. 2001).
The development of aripiprazole was guided by prevailing hypotheses of the etiology of schizophrenia. The dopamine hypothesis (Seeman and Niznik 1990) proposes that abnormalities in dopaminergic neurotransmission in the brain cause the symptoms of schizophrenia and suggests that schizophrenia involves a biphasic disturbance in dopaminergic pathways (Davis et al. 1991; Pycock et al. 1980; Weinberger 1987). Underactivity of the mesocortical dopaminergic pathway leads to hypodopaminergic activity in the frontal cortex, whereas overactivity in the mesolimbic pathway causes increased dopaminergic neurotransmission. The latter is presumed to cause positive or psychotic symptoms, while the former is believed to underlie negative symptoms and cognitive impairment. Another influential hypothesis suggests that the activity of dopaminergic pathways is modulated by serotonergic neurons. In the striatum, serotonin (5-hydroxytryptamine; 5-HT) release inhibits dopamine, while in the frontal cortex it has a modulatory effect on pyramidal neurons and can affect glutamate release.
Most typical and atypical antipsychotic agents behave as full D2 receptor antagonists. Their actions in the mesolimbic pathway would therefore be expected to benefit patients with schizophrenia by reducing positive symptoms. D2 receptor antagonism in the other dopaminergic pathways, however, would be expected to cause unwanted side effects, including exacerbation of negative symptoms (mesocortical pathways), EPS and tardive dyskinesia (nigrostriatal tract), and hyperprolactinemia (tuberoinfundibular pathway) (Figure 29–1).
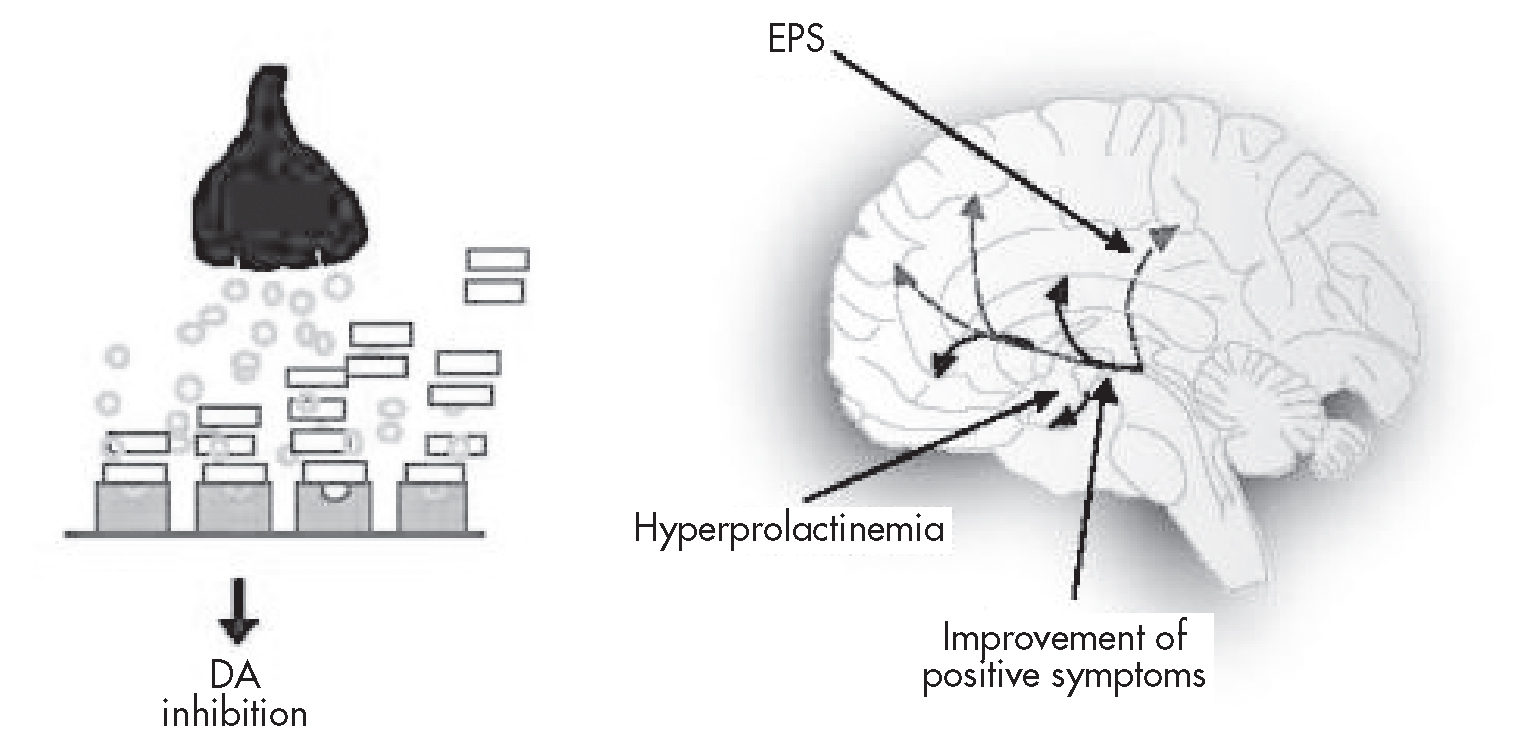
FIGURE 29–1. Conventional dopamine (DA) antagonist activity: effect on positive symptoms, extrapyramidal side effects (EPS), and prolactin levels.
The serotonin hypothesis may explain why the atypical agents, which have antagonist activity at 5-HT2A receptors, are associated with fewer EPS and do not exacerbate (and, in fact, partially alleviate) negative symptoms and cognitive impairment (Leysen et al. 1993; Millan 2000; Rao and Möller 1994; Richelson 1999).
On the basis of aripiprazole’s pharmacodynamic profile—partial agonist activity (rather than full antagonist activity) at both dopaminergic (D2; Burris et al. 2002) and serotonergic (5-HT1A; Jordan et al. 2002) receptors, and full antagonist activity at 5-HT2A receptors (McQuade et al. 2002)—it was anticipated that aripiprazole treatment would be associated with a reduced burden of unwanted D2 antagonist activity in the mesocortical, nigrostriatal, and tuberoinfundibular pathways—the activity associated with some of the side effects of typical and atypical antipsychotic agents (Figure 29–2).
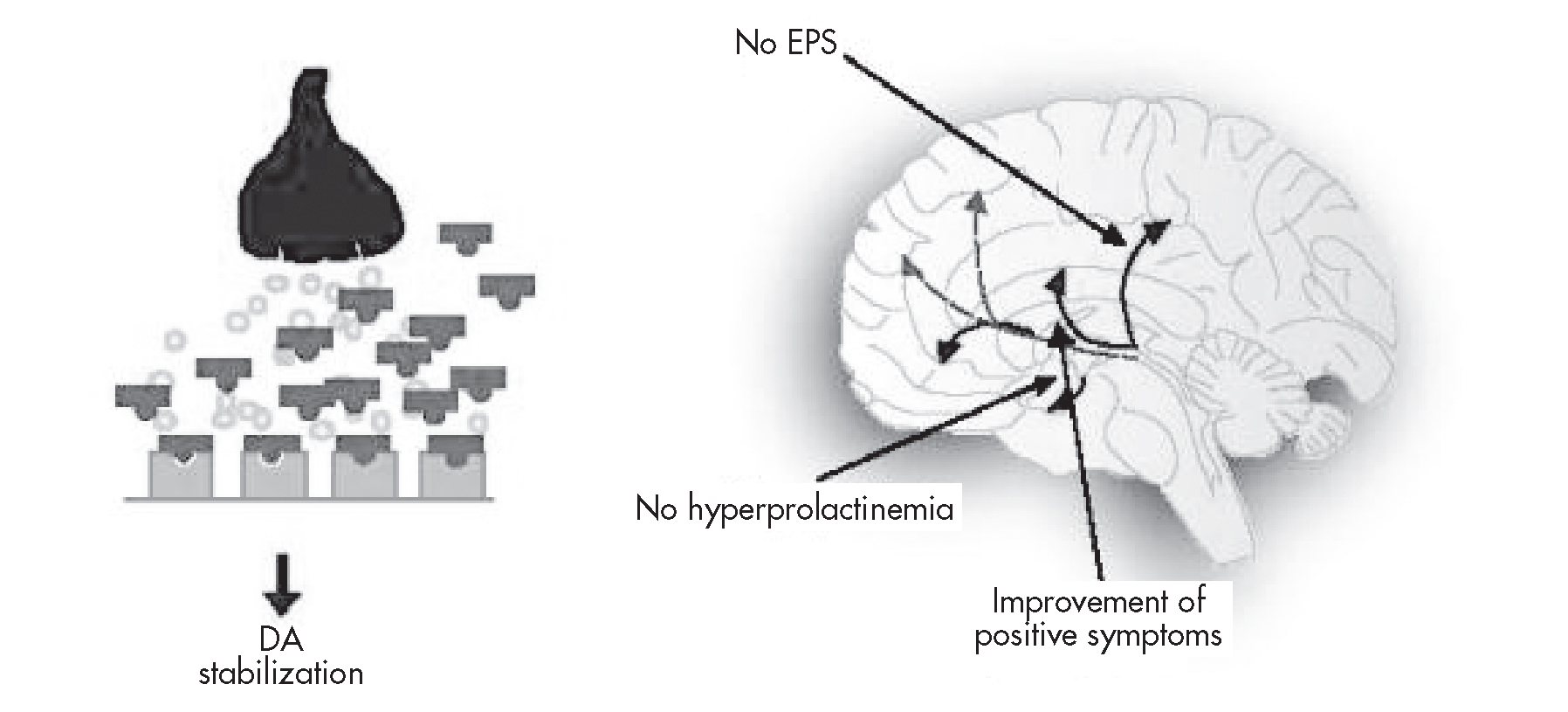
FIGURE 29–2. Dopamine (DA) partial agonist activity: effect on positive symptoms, extrapyramidal side effects (EPS), and prolactin levels.
Brexpiprazole’s reduced intrinsic activity at D2 receptors and stronger antagonism at 5-HT2A receptors relative to aripiprazole suggest a lower potential to induce D2 partial agonist–mediated effects (i.e., akathisia, insomnia, nausea, and restlessness) and D2 antagonist effects (i.e., dystonias, tardive dyskinesia, and hyperprolactinemia) (Fleischhacker 2005; Maeda et al. 2014a).
Structure–Activity Relations and Pharmacological Profile
Aripiprazole
Aripiprazole—7-{4-(4-[2,3-dichlorophenyl]-1-piperazinyl)butoxy}-3,4-dihydrocarbostyril, a dihydroquinolinone (Figure 29–3)—exhibits potent partial agonist activity at D2 (Burris et al. 2002) and 5-HT1A (Jordan et al. 2002) receptors, together with potent antagonist activity at 5-HT2A receptors. It also has high affinity for D3 receptors; moderate affinity for dopamine4 (D4), serotonin2C (5-HT2C), serotonin7 (5-HT7), α1-adrenergic, and histamine1 (H1) receptors and the serotonin transporter (SERT); and negligible affinity for cholinergic muscarinic receptors (Table 29–1). The active metabolite of aripiprazole, dehydroaripiprazole, exhibits a similar affinity at D2 receptors and has not been shown to have a pharmacological profile that is clinically significantly different from that of the parent compound.
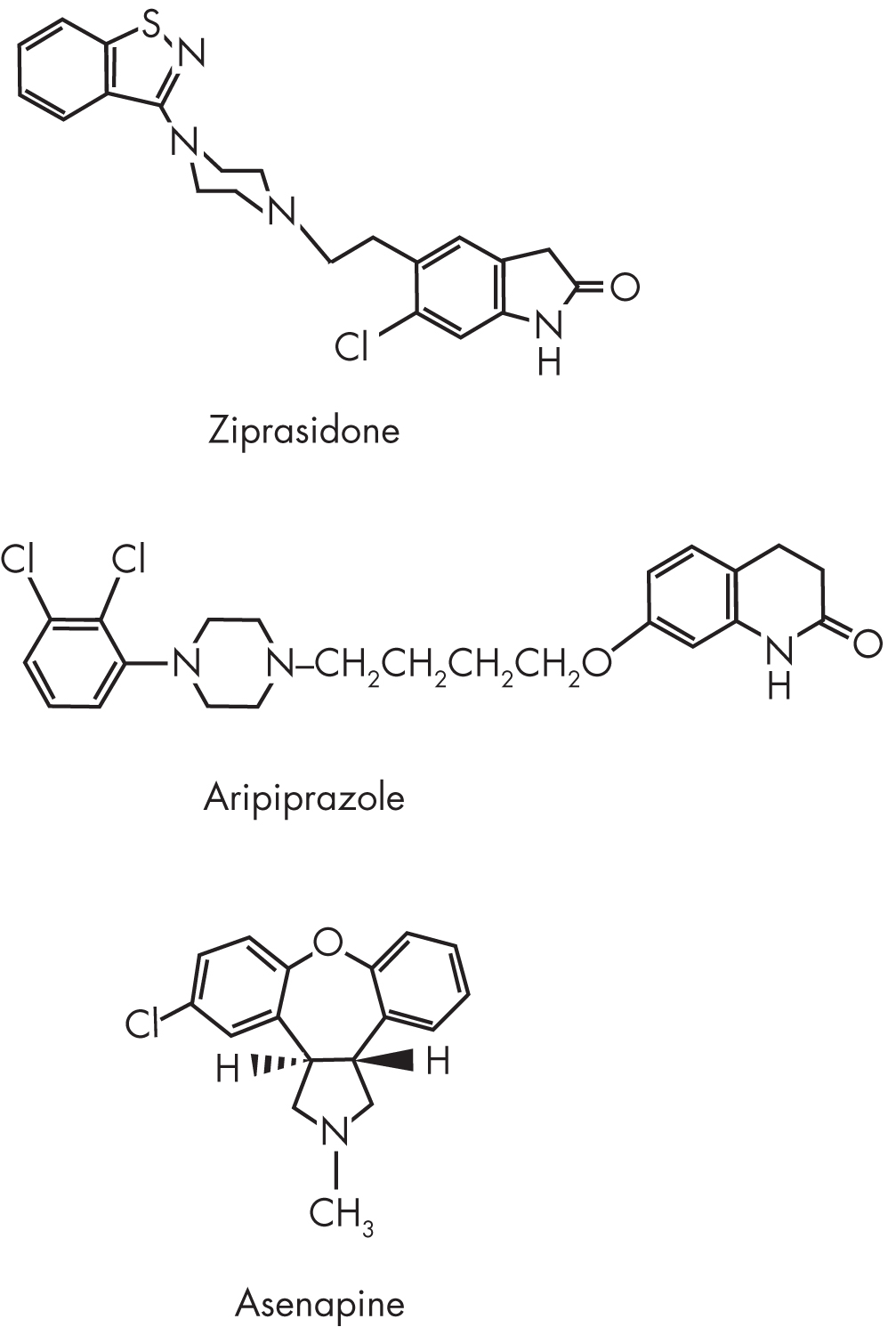
FIGURE 29–3. Chemical structure of aripiprazole.
Receptor type |
Ki (nM) |
Dopaminergic |
|
D1 |
265 |
D2a |
0.34 |
D3 |
0.8 |
D4 |
44 |
D5 |
95 |
Serotonergic |
|
5-HT1Ab |
1.7 |
5-HT2A |
3.4 |
5-HT2C |
15 |
5-HT6 |
214 |
5-HT7 |
39 |
SERT |
98 |
Histaminic |
|
H1 |
61 |
Adrenergic |
|
α1c |
57 |
Muscarinic |
IC50 (nM) |
M1c |
>1,000 |
Note. SERT=serotonin transporter. Source. Adapted from McQuade RD, Burris KD, Jordan S, et al.: “Aripiprazole: A Dopamine-Serotonin System Stabilizer.” International Journal of Neuropsychopharmacology 5 (Suppl 1):S176, 2002, with the following exceptions: aBurris KD, Molski TF, Xu C, et al.: “Aripiprazole, A Novel Antipsychotic, Is a High Affinity Partial Agonist at Human Dopamine D2 Receptors.” Journal of Pharmacology and Experimental Therapeutics 302:381–389, 2002. bJordan S, Koprivica V, Chen R, et al.: “The Antipsychotic Aripiprazole Is a Potent, Partial Agonist at the Human 5-HT1A Receptor.” European Journal of Pharmacology 441:137–140, 2002. cAbilify (Aripiprazole) Tablets: U.S. Full Prescribing Information. Tokyo, Japan, Otsuka Pharmaceutical Co., February 2012. |
|
Brexpiprazole
Brexpiprazole—7-{4-[4-(1-benzothiophen-4-yl)piperazin-1-yl]butoxy}quinolin-2(1H)-one (Figure 29–4)—exhibits potent partial agonist properties at 5-HT1A, D2, and D3 receptors. This is combined with potent antagonist activity at 5-HT2A receptors and α1B/α2C adrenoceptors. It also displays antagonistic properties at serotonin2B (5-HT2B) and serotonin7A (5-HT7A) receptors and moderate-potency antagonism at H1 receptors. Brexpiprazole has negligible affinity for cholinergic muscarinic receptors (Table 29–2) (Maeda et al. 2014a; Otsuka Pharmaceutical 2015).
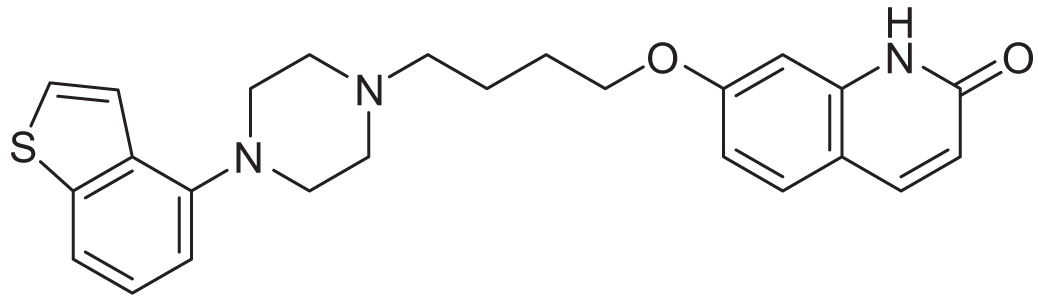
FIGURE 29–4. Chemical structure of brexpiprazole.
Receptor type |
Ki (nM) |
Dopaminergic |
|
D2 |
0.30 |
D3 |
1.1 |
Serotonergic |
|
5-HT1A |
0.12 |
5-HT2A |
0.47 |
5-HT2B |
1.9 |
5-HT7a |
3.7 |
Histaminic |
|
H1 |
19 |
Adrenergic |
|
α1Aa |
3.8 |
α1B |
0.17 |
α1Da |
2.6 |
α2C |
0.59 |
Muscarinic |
|
M1 |
67% at 10 μM |
Source. Adapted from Maeda K, Sugino H, Akazawa H, et al.: “Brexpiprazole I: In Vitro and In Vivo Characterization of a Novel Serotonin-Dopamine Activity Modulator.” J Pharmacol Exp Ther 350:589–604, 2014, with the following exception: aRexulti (Brexpiprazole) Tablets: U.S. Full Prescribing Information. Tokyo, Japan, Otsuka Pharmaceutical Co., August 2015. |
|
Pharmacokinetics and Disposition
Aripiprazole
Aripiprazole is available for oral administration as tablets in strengths of 2, 5, 10, 15, 20, and 30 mg. The effective dosage range is 10–30 mg/day for schizophrenia patients and 15–30 mg/day for bipolar I disorder patients. For adjunctive treatment of major depressive disorder (MDD) and irritability associated with autism spectrum disorder, the recommended dosage range is 2–15 mg/day. For Tourette’s disorder, body weight–based dosing of 2–10 mg/day for patients weighing less than 50 kg and 2–20 mg/day for patients weighing 50 kg or more is indicated. Aripiprazole is taken once daily with or without food and is well absorbed after oral administration, with peak plasma concentrations occurring within 3–5 hours. Absolute oral bioavailability is 87%. In plasma, aripiprazole and its major metabolite, dehydroaripiprazole, are both more than 99% bound to proteins, primarily albumin. Aripiprazole is extensively distributed outside the vascular system, and human studies demonstrating dose-dependent occupancy of D2 receptors have confirmed that aripiprazole penetrates the brain. Elimination half-lives for aripiprazole and dehydroaripiprazole are 75 hours and 94 hours, respectively (Otsuka Pharmaceutical 2016a).
Of note, several aripiprazole formulations were withdrawn from the market by the manufacturer in 2015 for reasons unrelated to efficacy, safety, or tolerability. Discontinued formulations include the aripiprazole oral disintegrating tablet at 10-mg and 15-mg strengths, the oral solution, and the short-acting intramuscular injection.
In February 2013, the U.S. Food and Drug Administration (FDA) approved a long-acting injectable formulation of aripiprazole for the treatment of schizophrenia. The effective dosage range is 300–400 mg once monthly. It is recommended that oral aripiprazole supplementation (at 10–20 mg/day) or an alternative antipsychotic be continued for 2 weeks after the first administered injection of long-acting aripiprazole. The aripiprazole depot formulation consists of a lyophilized powder of unmodified aripiprazole. The depot formulation is reconstituted in water and injected into the gluteal muscle. The median peak plasma concentration is reached after 5–7 days. The mean terminal half-lives of the 300-mg and 400-mg doses are 29.9 and 46.5 days, respectively. Steady-state plasma concentration is attained after the fourth dose (Otsuka Pharmaceutical 2016b). The aripiprazole once-monthly dose of 400 mg with supplemental oral aripiprazole 10 mg/day for the first 2 weeks produces a pharmacokinetic profile consistent with multiple daily dosing of aripiprazole 10–30 mg, with a maximum plasma concentration comparable to the 30 mg/day dosage and a minimum plasma concentration comparable to the 10 mg/day dosage (Mallikaarjun et al. 2013).
In October 2015, the FDA approved aripiprazole lauroxil, a second long-acting injectable formulation of aripiprazole, for the treatment of schizophrenia. The effective dosages (441 mg, 662 mg, and 882 mg once monthly) correlate with oral aripiprazole dosages of 10 mg, 20 mg, and 30 mg/day, respectively. Aripiprazole lauroxil 882 mg may be administered at a dosing interval up to 6 weeks. The recommended period of oral aripiprazole supplementation is 3 weeks after the first administered injection.
Aripiprazole lauroxil, a prodrug of aripiprazole, initially undergoes enzyme-mediated hydrolysis to N-hydroxymethyl aripiprazole and then undergoes water-mediated hydrolysis to aripiprazole, the active form. After a single intramuscular injection, aripiprazole appears in systemic circulation within 5–6 days. Aripiprazole reaches steady state after four consecutive monthly injections. Mean terminal half-life ranges from 29.2 to 34.9 days (Alkermes 2015).
Aripiprazole is metabolized primarily in the liver. Two hepatic cytochrome P450 (CYP) enzymes, 2D6 and 3A4, catalyze its dehydrogenation to dehydroaripiprazole. Therefore, coadministration of aripiprazole with inducers or inhibitors of these CYP enzymes may require dosage adjustment. The active metabolite accounts for 40% of drug exposure, but the predominant circulating moiety is the parent drug. Aripiprazole does not undergo direct glucuronidation and is not a substrate for the following CYP enzymes: 1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, and 2E1. Interactions with inhibitors or inducers of these enzymes, or with chemicals related to cigarette smoke, are therefore unlikely to occur.
Brexpiprazole
Brexpiprazole is available for oral administration as tablets in strengths of 0.25, 0.5, 1, 2, and 3 mg. The effective dosage range is 2–4 mg/day for treatment of schizophrenia and 1–3 mg/day for adjunctive treatment of MDD. Brexpiprazole is taken once daily with or without food and is well absorbed after oral administration, with peak plasma concentrations occurring within 4 hours. Absolute oral bioavailability is 95%. Brexpiprazole is highly bound to serum albumin and α1-acid glycoprotein (>99%). Elimination half-lives for brexpiprazole and DM-3411, its major metabolite, are 91 hours and 86 hours, respectively (Otsuka Pharmaceutical 2015).
Brexpiprazole is metabolized primarily through the liver by CYP enzymes 2D6 and 3A4. Therefore, coadministration of brexpiprazole with inducers or inhibitors of these CYP enzymes may require dosage adjustment. At steady state (which is reached in 10–12 days), the inactive metabolite DM-3411 represents 23%–48% of brexpiprazole AUC (area under the time–concentration curve) in plasma (Otsuka Pharmaceutical 2015).
Mechanism of Action
Aripiprazole and brexpiprazole both have partial agonist activity at D2 receptors. The activity of aripiprazole and brexpiprazole at D2 receptors has been studied in animal models of schizophrenia (Kikuchi et al. 1995; Maeda et al. 2014b). In the intact rat with repetitive stereotyped behavior (stereotypy) induced by apomorphine, aripiprazole and brexpiprazole inhibit stereotypy and locomotion (Kikuchi et al. 1995; Maeda et al. 2014a). These agents may therefore be expected to inhibit hyperdopaminergic activity in the mesolimbic pathway of patients with schizophrenia, thereby (like other available agents) exerting antipsychotic action against the positive symptoms of schizophrenia. On the other hand, in animal models of hypodopaminergic activity, such as the reserpinized rat, aripiprazole and brexpiprazole have D2 receptor agonist activity (Maeda et al. 2014b). Because aripiprazole and brexpiprazole may exert either D2 antagonist activity under hyperdopaminergic conditions or D2 agonist activity under hypodopaminergic conditions, they may be less likely than other antipsychotics to cause excessive D2 receptor antagonism. In preclinical studies, brexpiprazole showed lower intrinsic activity at D2 receptors, which suggests a reduced propensity to cause D2 agonist–associated side effects such as nausea, insomnia, and akathisia (Maeda et al. 2014a, 2014b). It is possible that this lower activity may diminish the effects of excess D2 receptor antagonism, including EPS. No comparative clinical trials of aripiprazole and brexpiprazole have yet been conducted.
Both aripiprazole and brexpiprazole may offer further therapeutic benefits through modulation of central serotonergic pathways. Preclinical studies showed that aripiprazole and brexpiprazole have antagonist activity at 5-HT2A receptors (Maeda et al. 2014b; McQuade et al. 2002), a characteristic that has been associated with reductions in EPS (Meltzer 1999) and in negative symptoms. In vitro studies also have shown that both aripiprazole and brexpiprazole have partial agonist activity at 5-HT1A receptors (Jordan et al. 2002; Maeda et al. 2014b), a feature that has been associated with improvement in negative, cognitive, depressive, and anxiety symptoms (Millan 2000).
The side effects of nausea/vomiting may be explained by the dopamine agonist effects of aripiprazole and brexpiprazole, whereas orthostatic hypotension and mild sedation/weight gain are likely related to these agents’ antagonist activity at α1-adrenergic and H1 receptors, respectively.
Aripiprazole lauroxil is a prodrug of aripiprazole. It initially undergoes enzyme-mediated hydrolysis to N-hydroxymethyl aripiprazole and then undergoes water-mediated hydrolysis to aripiprazole, the active form (Alkermes 2015).
Indications and Efficacy
Aripiprazole
In the United States, aripiprazole is approved by the FDA for the following indications: treatment of schizophrenia in adults and in adolescents ages 13–17 years; acute treatment of manic or mixed episodes associated with bipolar I disorder as monotherapy and as an adjunct to lithium or valproate in adults and pediatric patients ages 10–17 years; maintenance treatment of manic or mixed episodes associated with bipolar I disorder as monotherapy and as an adjunct to lithium or valproate in adults; use as an adjunct to antidepressant treatment in adults with MDD who have had an inadequate response to antidepressant therapy; treatment of irritability associated with autism spectrum disorder in pediatric patients ages 6–17 years; and treatment of Tourette’s disorder in pediatric patients ages 6–18 years. Additionally, intramuscular aripiprazole injection is approved for the acute treatment of agitation associated with schizophrenia or bipolar disorder (manic or mixed) in adults, and currently is available in two formulations for long-acting injectable depot maintenance and relapse management of schizophrenia in adults. The previously available formulations Abilify Discmelt orally disintegrating tablet (10 mg and 15 mg), Abilify oral solution (1 mg/mL), and Abilify short-acting intramuscular injection (9.75 mg/1.3 mL) were voluntarily withdrawn from the U.S. market in 2015.
Schizophrenia
The efficacy of aripiprazole in the treatment of an acute symptom relapse in schizophrenia was demonstrated in four short-term (4-week) double-blind, placebo-controlled studies. Among these was a pivotal Phase III parallel-group multicenter study with four treatment arms comparing aripiprazole (15 or 30 mg/day) with placebo (Kane et al. 2002). Aripiprazole at either dosage produced statistically significant improvements from baseline on standard psychometric scales by week 2. This trial suggested that at daily dosages of 15 mg and 30 mg, aripiprazole provides effective symptom control in patients experiencing an acute exacerbation of schizophrenia symptoms.
In another short-term multicenter Phase III study involving acute symptom relapse in schizophrenia or schizoaffective disorder (Potkin et al. 2003), patients were randomly assigned to receive aripiprazole 20 mg/day, aripiprazole 30 mg/day, risperidone 6 mg/day, or placebo for 4 weeks. Compared with placebo, aripiprazole (at both dosages) and risperidone produced statistically significant improvements in scores on standard scales designed to measure antipsychotic efficacy.
The antipsychotic efficacy of aripiprazole in acute symptom relapse in schizophrenia was also demonstrated in two Phase II dose-ranging studies. Patients were randomly assigned to receive aripiprazole 2 mg, 10 mg, or 30 mg/day or haloperidol 10 mg/day (Daniel et al. 2000). All three dosages of aripiprazole produced improvements from baseline on efficacy measures, and the 30-mg/day dosage produced statistically significant improvement compared with placebo on all illness scores. Similarly, in a Phase II dosage titration study, aripiprazole 5–30 mg/day was superior to placebo in improving Brief Psychiatric Rating Scale (BPRS) Total, BPRS Core, Clinical Global Impression–Severity (CGI-S), and Positive and Negative Syndrome Scale (PANSS)–Total scores (Petrie et al. 1997).
Results from the three 4-week fixed-dosage studies discussed above were pooled for analysis with those from an additional 6-week placebo-controlled, fixed-dosage study of aripiprazole at 10 mg, 15 mg, or 20 mg/day (Lieberman et al. 2002). The pooled analysis, involving 898 patients randomly assigned to receive aripiprazole, showed that at all investigated dosages greater than 2 mg/day, aripiprazole exhibited antipsychotic efficacy superior to that of placebo. Onset of effect was rapid, with improvement on psychometric scores detectable within 1 week of starting treatment. These pooled efficacy results demonstrate that dosages of 10–30 mg/day represent an effective therapeutic range for aripiprazole treatment.
Two long-term double-blind, randomized controlled multicenter trials yielded further confirmation of aripiprazole’s efficacy. A 26-week placebo-controlled study in patients with chronic stable schizophrenia investigated the efficacy of aripiprazole 15 mg/day in relapse prevention (Pigott et al. 2003). Aripiprazole treatment significantly increased the time to relapse and resulted in significantly fewer relapses at endpoint compared with placebo (34% vs. 57%). From week 6 of therapy, PANSS–Total and PANSS–Positive subscale scores were significantly more improved with aripiprazole than with placebo.
In a 52-week study (Kasper et al. 2003), patients with schizophrenia who were experiencing an acute symptom relapse were randomly assigned to receive aripiprazole 30 mg/day or haloperidol 10 mg/day. Significantly more aripiprazole-treated patients than haloperidol-treated patients were still taking the medication and responding to treatment at weeks 8, 26, and 52. Both treatments produced sustained improvements from baseline on PANSS–Total and PANSS–Positive subscale scores. However, aripiprazole produced significantly greater improvements in negative and depressive symptoms at weeks 26 and 52 and was associated with significantly lower scores on all EPS assessments compared with haloperidol.
The efficacy of aripiprazole monotherapy in antipsychotic-resistant schizophrenia was evaluated in a 6-week double-blind, randomized trial in patients whose symptoms had not improved during a prospective 4- to 6-week open trial with either olanzapine or risperidone (Kane et al. 2007). Subjects were randomly assigned to receive aripiprazole (15–30 mg/day) or perphenazine (8–64 mg/day). After 6 weeks, there was no statistical difference between the two groups on efficacy measures. However, compared with aripiprazole, perphenazine was associated with higher rates of EPS and serum prolactin elevations.
The efficacy of aripiprazole in the treatment of schizophrenia in pediatric patients (ages 13–17 years) was evaluated in a 6-week placebo-controlled outpatient trial comparing two fixed daily dosages of aripiprazole (10 mg or 30 mg) with placebo (Findling et al. 2008). Both aripiprazole dosages demonstrated statistically significant differences from placebo in reductions in PANSS–Total score; the 30-mg/day dosage was not shown to be more efficacious than the 10-mg/day dosage. Adverse events occurring in more than 5% of either aripiprazole group and with a combined incidence at least twice the rate for placebo were EPS, somnolence, and tremor. Mean body weight changes were −0.8, 0.0, and +0.2 kg for placebo, aripiprazole 10 mg/day, and aripiprazole 30 mg/day, respectively.
Two studies have investigated the efficacy of aripiprazole long-acting depot injection in the prevention of relapse in schizophrenia. In the first investigation, a 52-week randomized, placebo-controlled, long-term multicenter maintenance study, subjects requiring chronic treatment with an antipsychotic entered an oral aripiprazole stabilization phase followed by an intramuscular depot conversion and stabilization phase. Those patients meeting stabilization criteria for 12 consecutive weeks were then randomly assigned to receive aripiprazole 400-mg long-acting depot or placebo depot for the 52-week double-blind maintenance phase of the study. The primary outcome measure was time to relapse, defined as meeting any or all of the following criteria at any time during the maintenance phase: 1) clinical worsening (defined as Clinical Global Impression–Improvement [CGI-I] score ≥5 plus increase in any core PANSS items), 2) hospitalization, 3) risk of suicide, or 4) violent behavior. The aripiprazole group showed a significantly lower rate of relapse compared with the placebo group (9.6% vs. 36.8%, respectively) (Kane et al. 2012). The second investigation was a 38-week double-blind noninferiority study that compared relapse rates for aripiprazole once-monthly 400-mg depot injection, oral aripiprazole (10–30 mg/day), and aripiprazole once-monthly 50-mg depot injection, using criteria similar to those used in the Kane et al. study discussed above. There was no significant difference in rate of relapse with the once-monthly 400-mg depot formulation (7.12%) versus the oral formulation (10–30 mg/day), and both were significantly superior to the 50-mg depot formulation (21.8%; P<0.0001) (Fleischhacker et al. 2014).
One 12-week randomized, double-blind, placebo-controlled Phase III multicenter trial evaluated the efficacy of aripiprazole once-monthly injection for the management of acute exacerbations in chronic schizophrenia. Patients were randomly assigned to either the aripiprazole once-monthly 400-mg group with concomitant oral aripiprazole (mean daily dosage=12.8 mg) for 2 weeks (n=168) or the placebo group (n=172). The aripiprazole group showed sustained improvements in PANSS–Total scores, PANSS–Positive and –Negative subscale scores, and CGI-I scores, with improvements apparent within the first week of administration (Kane et al. 2014).
An open-label mirror-image multicenter study in a naturalistic community setting (Kane et al. 2013) compared total psychiatric hospitalization rates in 183 patients (18–65 years) who had previously been treated with oral antipsychotics (retrospective phase) and who were then switched to the aripiprazole once-monthly depot formulation for 6 months (prospective phase). The rate of hospitalization for the 6-month prospective phase (14.2%) was found to be lower than the rate for the 6-month retrospective oral antipsychotic period (41.5%; P<0.0001). Additionally, the all-cause discontinuation rate for the prospective phase was high (44.8%), with 26 patients discontinuing because of adverse events including “psychiatric disorders” (e.g., worsening of psychoses, increased paranoia, agitation, anxiety, decreased self-care; 20 patients) and “nervous-system disorders” (akathisia; 2 patients). For all patients receiving at least one dose of aripiprazole once monthly (n=181), the most common treatment-emergent adverse events (occurring in ≥5% of patients) were psychotic disorder (7.7%), akathisia (7.2%), and insomnia (7.2%) (Kane et al. 2013).
Meltzer et al. (2015) conducted a 12-week double-blind, placebo-controlled multicenter study comparing once-monthly administration of aripiprazole lauroxil 441 mg or 882 mg versus placebo for 12 weeks. Statistically significant improvement was observed on the PANSS–Total score from baseline to day 85 in both medication groups, with placebo-adjusted differences of −10.9 (P< 0.001) and −11.9 (P<0.001) for aripiprazole lauroxil 441 mg and 882 mg, respectively.
Bipolar Disorder
The efficacy of aripiprazole in the treatment of acute manic episodes was established in two 3-week placebo-controlled trials in hospitalized patients whose symptoms met DSM-IV (American Psychiatric Association 1994) criteria for bipolar I disorder with manic or mixed episodes (Keck et al. 2003; Sachs et al. 2006). Aripiprazole was superior to placebo in reducing the Young Mania Rating Scale (YMRS) Total score and the Clinical Global Impression–Bipolar (CGI-BP) Severity of Illness score. In a third large randomized, double-blind trial (Vieta et al. 2005), aripiprazole was compared with haloperidol in the treatment of acute bipolar mania over a 12-week period. Significantly more patients remained in treatment and were classified as responders (>50% reduction in YMRS score from baseline) at week 12 in the aripiprazole group (49.7%) than in the haloperidol group (28.4%). EPS adverse events were more frequent with haloperidol than with aripiprazole (62.7% vs. 24.0%).
The efficacy of adjunctive aripiprazole with concomitant lithium or valproate in the treatment of manic or mixed episodes was established in a 6-week placebo-controlled study with a 2-week lead-in mood stabilizer monotherapy phase in adult patients who met DSM-IV criteria for bipolar I disorder (Vieta et al. 2008). Adjunctive aripiprazole starting at 15 mg/day with concomitant lithium or valproate (in a therapeutic range of 0.6–1.0 mEq/L or 50–125 μg/mL, respectively) was found to be superior to lithium or valproate with adjunctive placebo on the basis of reductions in YMRS Total scores and CGI-BP Severity of Illness scores.
Aripiprazole monotherapy was evaluated in the treatment of nonpsychotic depressive episodes associated with bipolar I disorder. The results of two identically designed 8-week randomized, double-blind, placebo-controlled multicenter studies were reported by Thase et al. (2008). The primary outcome measure was mean change from baseline to week 8 (last observation carried forward [LOCF]) in the Montgomery-Åsberg Depression Rating Scale (MADRS) Total score. Although statistically significant differences were observed during weeks 1–6, there were no statistically significant differences in change in MADRS Total score between aripiprazole and placebo at week 8 in either study.
To evaluate the long-term effectiveness of aripiprazole in delaying relapse in bipolar I disorder, a trial was conducted in patients whose symptoms met DSM-IV criteria for bipolar I disorder with a recent manic or mixed episode (Keck et al. 2006). Patients whose condition had been stabilized while taking open-label aripiprazole and who had maintained a clinical response for at least 6 weeks were randomly assigned to receive aripiprazole or placebo for the 26-week, double-blind phase. Aripiprazole-treated patients had significantly fewer relapses than placebo-treated patients (25% vs. 43%). Aripiprazole was superior to placebo in delaying the time to manic relapse but did not differ from placebo in delaying time to depressive relapse. Significant weight gain (≥7% increase from baseline) was seen in 13% of the aripiprazole patients and none of the placebo patients.
The efficacy of aripiprazole in the treatment of bipolar I disorder in pediatric patients (ages 10–17 years) was evaluated in two studies. The first study was a 4-week double-blind, placebo-controlled trial of outpatients whose symptoms met DSM-IV criteria for bipolar I disorder manic or mixed episodes with or without psychotic features. The trial compared two fixed daily dosages of aripiprazole (10 mg or 30 mg). Both dosages of aripiprazole were superior to placebo as measured by change from baseline to week 4 on the YMRS Total score (Otsuka Pharmaceutical 2016a). The second study was a 30-week randomized, placebo-controlled study comparing fixed daily dosages of aripiprazole (10 mg and 30 mg) with placebo in the treatment of adolescent bipolar disorder (Findling et al. 2013). Two hundred ten youths (ages 10–17 years) with bipolar I disorder (manic or mixed) with or without psychotic features were randomly assigned to receive oral aripiprazole 10 mg/day, oral aripiprazole 30 mg/day, or placebo. Both dosages of aripiprazole were superior to placebo; at week 30, aripiprazole-treated patients demonstrated significantly greater improvement on YMRS Total scores compared with placebo-treated patients.
Acute Agitation
The efficacy of the injectable formulation of aripiprazole in controlling acute agitation was evaluated in three short-term (24-hour) randomized, double-blind, placebo-controlled studies in patients with schizophrenia (Andrezina et al. 2006; Tran-Johnson et al. 2007) and patients with bipolar disorder (manic or mixed) (Zimbroff et al. 2007). Aripiprazole injection was statistically superior to placebo (P<0.05) in all three studies, as measured by PANSS–Excited Component (PANSS-EC) scores. In the two studies in agitated patients with schizophrenia, injectable aripiprazole and intramuscular haloperidol were both superior to placebo. In the study in agitated patients with bipolar I disorder, aripiprazole injection and lorazepam injection were both superior to placebo.
Adjunctive Treatment of Major Depressive Disorder
The efficacy of aripiprazole in the adjunctive treatment of MDD was demonstrated in three short-term (6-week) placebo-controlled trials (Berman et al. 2007, 2009; Marcus et al. 2008). During prospective antidepressant treatment, patients received one of several antidepressants (escitalopram, fluoxetine, paroxetine controlled release, sertraline, or venlafaxine extended release), each with single-blind adjunctive placebo. Patients with incomplete response continued taking the antidepressant and were randomly assigned to receive double-blind adjunctive placebo or adjunctive aripiprazole (2–15 mg/day with the potent CYP2D6 inhibitor fluoxetine or paroxetine; 2–20 mg/day with all other antidepressants). In all three trials, the mean change in MADRS Total score was significantly greater with adjunctive aripiprazole than with adjunctive placebo. Akathisia and restlessness were significantly more frequent with adjunctive aripiprazole than with adjunctive placebo.
Fava et al. (2012) evaluated the efficacy of low-dosage aripiprazole added to antidepressant therapy in patients with MDD who had experienced an inadequate response to prior antidepressant treatment. Two hundred twenty-five patients were randomly assigned to receive adjunctive treatment with aripiprazole 2 mg/day or placebo. The pooled, weighted response difference between aripiprazole 2 mg/day and placebo was 5.6% (P=0.18; not significant [NS]); the difference on the MADRS was −1.51 (P=0.065; NS). Of note, there was no difference in rates of akathisia with low-dosage aripiprazole compared with placebo. Thus, a reasonable initial therapeutic approach may be to try a low-dosage (2 mg/day) augmentation of aripiprazole, in view of its better tolerability, and to increase the dosage to 5 mg/day, and if necessary to 10 or 15 mg/day, in the face of continued nonresponse, given that the efficacy of the higher dosage range was supported by robust evidence in three previous positive studies (Fava et al. 2012).
Irritability in Autism Spectrum Disorder
The efficacy of aripiprazole in the treatment of irritability associated with DSM-IV autistic disorder was established in two 8-week placebo-controlled trials in children and adolescents (ages 6–17 years). Efficacy was evaluated with two assessment scales: the Aberrant Behavior Checklist (ABC) and the CGI-I scale. In the first trial (Owen et al. 2009), pediatric patients with DSM-IV autistic disorder received aripiprazole (2–15 mg/day, based on clinical response) or placebo. Patients who received aripiprazole (at a mean daily dosage of 8.6 mg at the end of the 8-week treatment) demonstrated significantly improved scores on the ABC–Irritability (ABC-I) subscale and on the CGI-I scale compared with patients who received placebo. In the other trial (Marcus et al. 2009), three fixed dosages of aripiprazole (5, 10, or 15 mg/day) were compared with placebo. All three dosages of aripiprazole were associated with significantly improved scores on the ABC-I subscale compared with placebo.
The efficacy and safety of aripiprazole in the long-term maintenance treatment of pediatric patients with irritability associated with DSM-IV autistic disorder were evaluated in a double-blind, randomized, placebo-controlled multicenter study in patients ages 6–17 years. After an initial phase in which single-blind aripiprazole was flexibly dosed (2–15 mg/day; average dosage of 9.0 mg/day) for 13–26 weeks to establish a stable response (defined as a ≥25% decrease on the ABC-I subscale and a rating of “much improved” or “very much improved” on the CGI-I scale), patients were randomly assigned to receive either aripiprazole (n=41, 2–15 mg/day) or placebo (n=44) for 16 weeks or until relapse. The primary outcome measure was time from randomization to relapse. No statistically significant differences in time to relapse were seen between the drug and the placebo group (P=0.97, hazard ratio=0.57, number needed to treat=6) (Findling et al. 2014).
Tourette’s Disorder
The efficacy of aripiprazole in children and adolescents with Tourette’s disorder was established in two short-term trials. In the first study, a double-blind, randomized, placebo-controlled, flexible-dose multicenter trial, patients with Tourette’s disorder (n=61) ages 6–18 years were randomly assigned to receive either placebo or aripiprazole (2–20 mg/day) for 10 weeks. Aripiprazole-treated patients demonstrated significant reductions on Yale Global Tic Severity Scale scores compared with patients receiving placebo (−15.0 [8.4] and −9.6 [8.8], respectively; P=0.0196) (Yoo et al. 2013). The mean change in phonic tic scores favored the aripiprazole group, but there was no difference in motor tic scores. Treatment-emergent adverse events were not statistically significantly different. The mean dosage range was 11.0 mg/day (Yoo et al. 2013). In the second study, a Phase III randomized, double-blind, placebo-controlled multicenter trial in patients with a diagnosis of Tourette’s disorder (ages 7–17 years), patients first underwent a 3- to 42-day washout period (Sallee et al. 2014). A total of 133 patients were then randomly assigned to receive low-dosage aripiprazole (5 mg/day if < 50 kg, 10 mg/day if ≥50 kg), high-dosage aripiprazole (10 mg/day if <50 kg, 20 mg/day if ≥50 kg), or placebo for 8 weeks. Both the low-dosage and high-dosage aripiprazole groups showed significant improvement in Yale Global Tic Severity Scale and CGI–Tourette’s Syndrome Scale scores compared with the placebo group (Sallee et al. 2014).
Off-Label Use
De Deyn et al. (2005) compared the efficacy, safety, and tolerability of aripiprazole versus placebo in patients with psychosis associated with Alzheimer’s disease in a 10-week double-blind multicenter study. The initial aripiprazole dosage of 2 mg/day was titrated upward (to 5, 10, or 15 mg/day) according to efficacy and tolerability, and evaluations included the Neuropsychiatric Inventory (NPI) Psychosis subscale and the BPRS. Aripiprazole-treated patients showed significantly greater improvements from baseline in BPRS–Psychosis subscale and BPRS–Core subscale scores at endpoint compared with patients receiving placebo.
In another double-blind multicenter study (Mintzer et al. 2007), patients with psychosis associated with Alzheimer’s disease were randomly assigned to receive either placebo or aripiprazole 2, 5, or 10 mg/day. The primary efficacy measure was mean change from baseline to week 10 on the Neuropsychiatric Inventory–Nursing Home (NPI-NH) Psychosis subscale score. Aripiprazole 10 mg/day showed significantly greater improvements than placebo on all efficacy measures (NPI-NH Psychosis subscale, CGI-S, BPRS Total and Core, and Cohen-Mansfield Agitation Inventory [CMAI] scores). Aripiprazole 5 mg/day showed significant improvements versus placebo on BPRS and CMAI scores. Aripiprazole 2 mg/day was not efficacious. No antipsychotic is currently approved in the United States for treating the behavioral and psychotic symptoms that frequently accompany dementia, and all carry a bolded warning based on increased mortality observed in patients with dementia-related psychosis treated with these agents.
Nickel et al. (2006) conducted a double-blind, placebo-controlled study in individuals whose clinical presentation met DSM-III-R (American Psychiatric Association 1987) criteria for borderline personality disorder. Subjects were randomly assigned in a 1:1 ratio to receive either aripiprazole (15 mg/day) or placebo for 8 weeks. At endpoint, significant changes in scores on most scales of the Symptom Checklist–90—Revised (SCL-90-R), on the Hamilton Rating Scale for Depression (Ham-D), on the Hamilton Anxiety Scale (Ham-A), and on all subscales of the State-Trait Anger Expression Inventory were observed in subjects who received aripiprazole. The improvements observed at 8 weeks were maintained at 18-month follow-up (Nickel et al. 2007).
Tiihonen et al. (2007) conducted a study in which individuals whose symptoms met DSM-IV criteria for intravenous amphetamine dependence were randomly assigned to receive aripiprazole (15 mg/day), slow-release methylphenidate (54 mg/day), or placebo for 20 weeks. The study was terminated prematurely because of unexpected results in the interim analysis. Contrary to the hypothesized result, patients who received aripiprazole treatment had significantly more amphetamine-positive urine samples than did patients in the placebo group, and patients who received methylphenidate had significantly fewer amphetamine-positive urine samples than patients who received placebo. Studies in subjects with cocaine use disorder are ongoing.
In a 12-week double-blind, placebo-controlled multicenter trial (Anton et al. 2008) evaluating the efficacy of aripiprazole in patients with DSM-IV alcohol dependence, aripiprazole did not differ from placebo on the study’s primary efficacy measure, mean percentage of days abstinent.
Brexpiprazole
Brexpiprazole is currently approved in the United States for schizophrenia and for use as an adjunct to antidepressant treatment in adults with MDD who have had an inadequate response to antidepressant therapy (Otsuka Pharmaceutical 2015).
Schizophrenia
The efficacy of brexpiprazole in the treatment of acute symptom exacerbations in schizophrenia has been evaluated in two 6-week double-blind, placebo-controlled multicenter studies. In the first study, a Phase III multicenter trial (BEACON study), patients with acute schizophrenia were randomly assigned to receive aripiprazole (1 mg, 2 mg, or 4 mg/day) or placebo. Brexpiprazole 4 mg/day produced statistically significant reductions (treatment difference −6.47; P=0.0022) versus placebo on the PANSS–Total score. Brexpiprazole dosages of 1 mg/day and 2 mg/day also produced numerical improvements versus placebo, although the degree of improvement was not statistically significant (P>0.05) (Kane et al. 2015).
In another 6-week multicenter double-blind, placebo-controlled Phase III trial (VECTOR study), patients experiencing an acute schizophrenia relapse were randomly assigned to receive aripiprazole (0.25 mg, 2 mg, or 4 mg/day) or placebo. At 6 weeks, both the 2-mg/day and the 4-mg/day dosages of brexpiprazole had produced statistically significantly greater reductions on PANSS–Total scores compared with placebo (treatment difference −8.72 [P<0.0001] and −7.64 [P=0.0006] for 2 mg/day and 4 mg/day, respectively) (Correll et al. 2015).
Adjunctive Treatment of Major Depressive Disorder
The efficacy of brexpiprazole in the adjunctive treatment of MDD has been demonstrated in two short-term randomized, double-blind Phase III studies. In the first study (Thase et al. 2015b), patients who had not responded to three prior trials with antidepressants entered an 8-week prospective period of open-label treatment with an antidepressant. Patients who showed inadequate response (defined as a score of ≥14 on the 17-item Ham-D [Ham-D-17]; <50% reduction from baseline on the Ham-D-17 and the MADRS Total score; and a CGI-I score of ≥3) were randomly assigned to receive double-blind antidepressant plus brexpiprazole (2 mg/day) or antidepressant plus placebo. Adjunctive brexpiprazole 2 mg/day produced statistically significant reductions on MADRS Total scores versus adjunctive placebo (i.e., antidepressant monotherapy) per final protocol (−8.36 vs. −5.15, respectively; P=0.0002) (Thase et al. 2015b).
The second study (Thase et al. 2015a) also evaluated the efficacy and safety of brexpiprazole (1 mg and 3 mg/day) versus placebo in the adjunctive treatment of antidepressant-resistant MDD. The study’s design also included an 8-week single-blind prospective treatment phase in which patients received one antidepressant. Patients who had an inadequate response (defined as a Ham-D-17 score ≥14; <50% reduction from baseline on the Ham-D-17 Total score and the MADRS Total score; and a CGI score ≥3) were randomly assigned to receive double-blind antidepressant plus brexpiprazole (1 mg/day or 3 mg/day) or antidepressant plus placebo. The brexpiprazole 3 mg/day treatment group showed statistically significant improvement (assessed by mean reduction from baseline in MADRS Total score) versus the placebo group (−8.29 vs. −6.33, respectively; P=0.0079), whereas the numerical improvement in MADRS Total score was not statistically significant for brexpiprazole 1 mg/day versus placebo (−7.64; P=0.0079) (Thase et al. 2015a).
Side Effects and Toxicology
Aripiprazole
A pooled analysis of safety and tolerability data from the five short-term studies (Marder et al. 2003; Stock et al. 2002) showed that aripiprazole treatment was well tolerated. The most commonly reported adverse events with aripiprazole were headache, insomnia, agitation, and anxiety. The incidence of adverse events was similar in the aripiprazole and placebo groups. The adverse-event profile of aripiprazole did not vary according to patient characteristics of age, sex, and race/ethnicity, and no deaths were reported during the short-term studies. Data from the four fixed-dosage studies showed that somnolence was the only adverse event seen with aripiprazole that was possibly dose related. Objective rating scale assessments were used to measure changes in parkinsonian symptoms (Simpson-Angus Scale [SAS]), dyskinesias (Abnormal Involuntary Movement Scale [AIMS]), and akathisia (Barnes Akathisia Rating Scale [BARS]). SAS scores with aripiprazole did not differ significantly from those with placebo, whereas AIMS scores improved significantly from baseline with aripiprazole compared with placebo. Aripiprazole did not produce consistent dose-dependent changes in BARS scores. The rate of discontinuation due to adverse events was 7.3% (Otsuka Pharmaceutical 2016a). According to the product labeling for aripiprazole in the United States, treatment-emergent adverse events most commonly reported with aripiprazole (occurring in ≥10% of patients with an incidence greater than that with placebo) in short-term trials of patients with schizophrenia (up to 6 weeks) or bipolar disorder (up to 3 weeks), respectively, included headache (aripiprazole 30% vs. placebo 25%), anxiety (20% vs. 17%), insomnia (19% vs. 14%), nausea (16% vs. 12%), vomiting (12% vs. 6%), dizziness (11% vs. 8%), constipation (11% vs. 7%), dyspepsia (10% vs. 8%), and akathisia (10% vs. 4%). A 26-week trial in schizophrenia reported a similar adverse-event profile except for a higher incidence of tremor (aripiprazole 8% vs. placebo 2%).
The most frequently reported adverse events with aripiprazole injection were headache (aripiprazole 12% vs. placebo 7%), nausea (9% vs. 3%), dizziness (8% vs. 5%), and somnolence (7% vs. 4%). In the three aripiprazole injection trials, the drug’s safety profile was comparable to that of placebo regarding the incidence of EPS, akathisia, or dystonia. The incidence of akathisia-related adverse events with aripiprazole injection was 2% (vs. 0% for placebo), while the incidence of dystonia with aripiprazole injection was less than 1% (vs. 0% for placebo). In addition, the incidence of QTc prolongation was also comparable between aripiprazole injection and placebo.
The most common adverse events reported for the aripiprazole long-acting injectable formulation in the Kane et al. (2012) 52-week trial were insomnia (aripiprazole 10% vs. placebo 9%), tremor (5.9% vs. 1.5%), headache (5.9% vs. 5.2%), and akathisia (5.6% vs. 6%). In the Kane et al. (2014) 12-week acute management of schizophrenia study, the most common adverse events were increased weight (aripiprazole 16.8% vs. placebo 7%), headache (14.4% vs. 16.3%), and akathisia (11.4% vs. 3.5%). There was no significant difference in rates of EPS other than akathisia between the 400-mg aripiprazole group and the placebo group. In a 24-week open-label parallel-arm study conducted by Mallikaarjun et al. (2013), the tolerability and safety of aripiprazole once monthly in schizophrenia were comparatively evaluated for dosages of 200 mg, 300 mg, and 400 mg. The most common adverse events were injection-site pain (aripiprazole 400 mg, 28.6%), tremor (aripiprazole 400 mg, 21.4%; aripiprazole 300 mg, 6.7%), and vomiting (aripiprazole 300 mg, 13.3%; aripiprazole 400 mg, 14.3%).
The most common adverse events in a 12-week Phase III trial of aripiprazole lauroxil in the treatment of acute exacerbations of schizophrenia were akathisia, headache, insomnia, and injection-site pain (occurring in >5% of patients). Akathisia occurred at twice the rate of placebo for aripiprazole lauroxil 882 mg and 441 mg (4.3%, 11.6%, and 11.5%, respectively) (Meltzer et al. 2015). The majority (>75%) of akathisia episodes occurred within 3 weeks of receiving the first injection.
Minimal changes in mean body weight were observed with aripiprazole treatment in short-term studies (pooled data +0.71 kg) (Marder et al. 2003) and in long-term studies (26-week: −1.26 kg; 52-week: +1.05 kg) (Kasper et al. 2003; Pigott et al. 2003).
Olanzapine and aripiprazole were compared on their propensity to cause weight gain and other metabolic disturbances in a 26-week randomized, double-blind multicenter trial (McQuade et al. 2004). Statistically significant differences in mean weight change were observed between treatments beginning at week 1 and were sustained throughout the study. At week 26, there was a mean weight loss of 1.37 kg (3.04 lb) with aripiprazole compared with a mean weight gain of 4.23 kg (9.40 lb) with olanzapine among patients who continued with therapy (P<0.001). Changes in fasting plasma levels of total cholesterol, high-density lipoprotein cholesterol, and triglycerides were significantly different in the two treatment groups, with worsening of the lipid profile among patients treated with olanzapine.
Aripiprazole treatment was not associated with increases in prolactin levels during either short- or long-term studies. (In fact, prolactin levels were shown to be slightly decreased by aripiprazole.)
Overall, aripiprazole treatment is associated with a low incidence of EPS (other than akathisia) and EPS-related symptoms and with minimal or no effects on weight gain, QTc interval, or circulating levels of cholesterol, glucose, and prolactin. Treatment with aripiprazole may reduce the burden of antipsychotic-associated side effects, thereby leading to improved patient adherence and decreased risks of acute relapse.
Brexpiprazole
Overall, brexpiprazole has been well tolerated in the four short-term clinical trials conducted thus far, with lower rates of discontinuation due to adverse events compared with placebo. In pooled analyses for two trials of brexpiprazole in the adjunctive treatment of MDD, the rate of discontinuation due to adverse reactions for all dosages of brexpiprazole was 3%, compared with 1% for placebo (Otsuka Pharmaceutical 2015). In the two Phase III trials for brexpiprazole in acute schizophrenia, the overall rates of discontinuation due to adverse event were lower than those for placebo (Correll et al. 2015; Kane et al. 2015).
The most common side effects varied per available studies. In one Phase III trial in acute schizophrenia, the most common adverse events for brexpiprazole were headache, agitation, and insomnia, with rates of akathisia lower than those with placebo but with a dose-dependent increase for brexpiprazole (Correll et al. 2015). Comparatively, the most common adverse event in another Phase III trial was akathisia for brexpiprazole 2 mg (4.4%) and 4 mg (7.2%) versus placebo (2.2%), with akathisia most commonly occurring within the first 3 weeks of treatment. In two short-term trials for adjunctive use in MDD, pooled analysis showed that the most common adverse event was akathisia (8.6%) (Citrome 2015).
Pooled data from two short-term Phase III schizophrenia trials showed that 10% of brexpiprazole-treated patients had weight gains of 7% or greater from baseline during a 6-week trial, compared with 4.1% of patients receiving placebo. Similarly, the pooled data from both short-term MDD trials showed weight gain in the brexpiprazole group to be higher than that in the placebo group (6.7% vs. 1.9%), with the greatest increase being 1.6 kg for brexpiprazole 3 mg/day at 6 weeks (Citrome 2015; Otsuka Pharmaceutical 2015).
Change in prolactin level was studied in all treatment trials. In one Phase III trial of brexpiprazole in schizophrenia, there was no statistically significant change in prolactin level (Correll et al. 2015). In a second Phase III short-term trial of brexpiprazole in schizophrenia, the incidence of potentially clinically relevant prolactin values (one to two times the upper limit of normal) was highest in the brexpiprazole 4-mg/day group (19.1%) versus the placebo group (13.9%) (Kane et al. 2015). In a short-term Phase III trial of brexpiprazole in the adjunctive treatment of MDD, 0.4% of patients receiving brexpiprazole 3 mg/day, compared with 1.4% of patients receiving placebo, had a prolactin level greater than three times the upper limit of normal (Thase et al. 2015b).
The incidence of EPS was evaluated in all four short-term treatment trials by means of the BARS and the SAS. The percentage of patients treated with brexpiprazole plus an antidepressant who showed a shift from normal to abnormal was greater versus placebo for both the BARS (4% vs. 0.6%) and the SAS (4% vs. 3%). Similarly, the brexpiprazole group showed a greater shift from normal to abnormal versus placebo in both the BARS (2% vs. 1%) and the SAS (7% vs. 5%) in pooled data from both schizophrenia trials (Otsuka Pharmaceutical 2015).
With an overall minimal incidence of EPS beyond akathisia and minimal observed changes in QTc, lipid panel, and glucose panel values compared with placebo, brexpiprazole is another antipsychotic with a tolerable side-effect profile that may improve adherence long term (Correll et al. 2015; Kane et al. 2015; Thase et al. 2015a, 2015b).
Drug–Drug Interactions
Aripiprazole
Because aripiprazole is metabolized primarily by the hepatic CYP enzymes 2D6 and 3A4, it has the potential to interact with other substrates for these enzymes. Inducers of these enzymes may increase clearance and thereby reduce blood levels of aripiprazole, whereas inhibitors of CYP3A4 or CYP2D6 may inhibit elimination and thereby increase blood levels of aripiprazole. In vivo studies showed decreased levels of aripiprazole and dehydroaripiprazole in the plasma when aripiprazole was coadministered with carbamazepine, a CYP3A4 inducer. The aripiprazole dose should therefore be increased when the drug is administered concomitantly with carbamazepine. In vivo studies coadministering aripiprazole and ketoconazole (a CYP3A4 inhibitor) or quinidine (a CYP2D6 inhibitor) suggest that the aripiprazole dose should be reduced when aripiprazole is administered with strong 3A4 or 2D6 inhibitors. Aripiprazole exhibits α1-adrenergic receptor antagonist activity and therefore may enhance the effects of certain antihypertensive agents.
Brexpiprazole
Brexpiprazole is metabolized primarily by hepatic CYP enzymes 2D6 and 3A4; therefore, coadministration with inducers or inhibitors of these CYP enzymes requires dosage adjustments for brexpiprazole. In vivo studies showed increased levels of brexpiprazole when it was coadministered with ketoconazole (a CYP3A4 inhibitor) or quinidine (a CYP2D6 inhibitor), and it is therefore recommended that brexpiprazole dosages be reduced when brexpiprazole is coadministered with known CYP2D6 and CYP3A4 inhibitors. Plasma levels of brexpiprazole were decreased when the drug was coadministered with rifampin (a CYP3A4 inducer) during in vivo studies, and it is therefore recommended that brexpiprazole dosages be reduced when brexpiprazole is coadministered with known CYP3A4 inducers. Brexpiprazole has limited induction or inhibition of other CYP enzymes per multiple in vivo studies (Otsuka Pharmaceutical 2015).
Conclusion
Aripiprazole was the first agent that was not a full D2 receptor antagonist to show rapid and sustained antipsychotic activity, and it may be considered the first partial dopamine agonist combined with 5-HT-stabilizing properties. Short-term and long-term clinical trials in adult and pediatric patients with schizophrenia and bipolar I disorder have demonstrated that aripiprazole combines sustained antipsychotic and mood-stabilizing efficacy with an excellent safety and tolerability profile. Additional augmentation trials have confirmed the utility of aripiprazole in alleviating depressive symptomatology in patients with MDD who have not achieved adequate symptom relief with antidepressants alone. The efficacy and safety of aripiprazole have also been established in child and adolescent populations for the management of irritability in autism spectrum disorders and Tourette’s disorder. Aripiprazole is now also available in two different long-term depot injectable formulations for the management of schizophrenia in adults.
Brexpiprazole shares multiple similarities with aripiprazole, including partial dopamine agonism and 5-HT-stabilizing properties. However, brexpiprazole has lower intrinsic dopaminergic activity than does aripiprazole, which may indicate an even more favorable side-effect profile relative to the well-tolerated older drug. Several short-term clinical trials in adult patients with acute schizophrenia or with a lack of response to previous therapy for MDD have confirmed the efficacy and tolerability of this newly established atypical antipsychotic. A variety of clinical trials with brexpiprazole targeting different treatment populations, including head-to-head trials with other antipsychotics to further establish clinical efficacy, have been initiated or are ongoing.
In general, both aripiprazole and brexpiprazole are associated with low liability for EPS, QTc interval prolongation, prolactin elevation, weight gain, and disturbance of glucose or lipid metabolism. The combination of efficacy, safety, and tolerability suggests that both aripiprazole and brexpiprazole represent important options for the acute treatment of schizophrenia as well as for the adjunctive treatment of MDD. Aripiprazole additionally represents an important treatment option for bipolar I disorder, irritability in autism spectrum disorder, and Tourette’s disorder, and for long-term management of schizophrenia.
References
Alkermes: Aristada: Prescribing information. Waltham, MA, Alkermes, Inc., October 2015. Available at: https://www.aristada.com/downloadables/ARISTADA-PI.pdf. Accessed May 24, 2016.
Allison DB, Mentore JL, Heo M, et al: Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 156(11):1686–1696, 1999 10553730
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition, Revised. Washington, DC, American Psychiatric Association, 1987
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition. Washington, DC, American Psychiatric Association, 1994
Andrezina R, Josiassen RC, Marcus RN, et al: Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder: a double-blind, placebo-controlled comparison with intramuscular haloperidol. Psychopharmacology (Berl) 188(3):281–292, 2006 16953381
Anton RF, Kranzler H, Breder C, et al: A randomized, multicenter, double-blind, placebo-controlled study of the efficacy and safety of aripiprazole for the treatment of alcohol dependence. J Clin Psychopharmacol 28(1):5–12, 2008 18204334
Berman RM, Marcus RN, Swanink R, et al: The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 68(6):843–853, 2007 17592907
Berman RM, Fava M, Thase ME, et al: Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr 14(4):197–206, 2009 19407731
Burris KD, Molski TF, Xu C, et al: Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther 302(1):381–389, 2002 12065741
Citrome L: Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract 69(9):978–997, 2015 26250067
Correll CU, Skuban A, Ouyang J, et al: Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia: a 6-week randomized, double-blind, placebo-controlled trial. Am J Psychiatry 172(9):870–880, 2015 25882325
Daniel DG, Saha AR, Ingenito G, et al: Aripiprazole, a novel antipsychotic: overview of a phase II study result (abstract). Int J Neuropsychopharmacol 3 (suppl 1):S157, 2000
Davis KL, Kahn RS, Ko G, et al: Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 148(11):1474–1486, 1991 1681750
De Deyn P, Jeste DV, Swanink R, et al: Aripiprazole for the treatment of psychosis in patients with Alzheimer’s disease: a randomized, placebo-controlled study. J Clin Psychopharmacol 25(5):463–467, 2005 16160622
Fava M, Mischoulon D, Iosifescu D, et al: A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychother Psychosom 81(2):87–97, 2012 22286203
Findling RL, Robb A, Nyilas M, et al: A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry 165(11): 1432–1441, 2008 18765484
Findling RL, Correll CU, Nyilas M, et al: Aripiprazole for the treatment of pediatric bipolar I disorder: a 30-week, randomized, placebo-controlled study. Bipolar Disord 15(2):138–149, 2013 23437959
Findling RL, Mankoski R, Timko K, et al: A randomized controlled trial investigating the safety and efficacy of aripiprazole in the long-term maintenance treatment of pediatric patients with irritability associated with autistic disorder. J Clin Psychiatry 75(1):22–30, 2014 24502859
Fleischhacker WW: Aripiprazole. Expert Opin Pharmacother 6(12):2091–2101, 2005 16197361
Fleischhacker WW, Sanchez R, Perry PP, et al: Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry 205(2):135–144, 2014 24925984
Glassman AH, Bigger JT Jr: Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry 158(11):1774–1782, 2001 11691681
Jordan S, Koprivica V, Chen R, et al: The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur J Pharmacol 441(3):137–140, 2002 12063084
Kane JM, Carson WH, Saha AR, et al: Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 63(9):763–771, 2002 12363115
Kane JM, Meltzer HY, Carson WH Jr, et al; Aripiprazole Study Group: Aripiprazole for treatment-resistant schizophrenia: results of a multicenter, randomized, double-blind, comparison study versus perphenazine. J Clin Psychiatry 68(2):213–223, 2007 17335319
Kane JM, Sanchez R, Perry PP, et al: Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 73(5):617–624, 2012 22697189
Kane JM, Sanchez R, Zhao J, et al: Hospitalisation rates in patients switched from oral anti-psychotics to aripiprazole once-monthly for the management of schizophrenia. J Med Econ 16(7):917–925, 2013 23663091
Kane JM, Peters-Strickland T, Baker RA, et al: Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 75(11):1254–1260, 2014 25188501
Kane JM, Skuban A, Ouyang J, et al: A multicenter, randomized, double-blind, controlled phase 3 trial of fixed-dose brexpiprazole for the treatment of adults with acute schizophrenia. Schizophr Res 164(1–3):127–135, 2015 25682550
Kasper S, Lerman MN, McQuade RD, et al: Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychopharmacol 6(4):325–337, 2003 14609439
Keck PE Jr, Marcus R, Tourkodimitris S, et al; Aripiprazole Study Group: A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 160(9):1651–1658, 2003 12944341
Keck PE Jr, Calabrese JR, McQuade RD, et al; Aripiprazole Study Group: A randomized, double-blind, placebo-controlled 26-week trial of aripiprazole in recently manic patients with bipolar I disorder. J Clin Psychiatry 67(4):626–637, 2006 16669728
Kikuchi T, Tottori K, Uwahodo Y, et al: 7-(4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butyloxy)-3,4-dihydro-2(1H)-quinolinone (OPC-14597), a new putative antipsychotic drug with both presynaptic dopamine autoreceptor agonistic activity and postsynaptic D2 receptor antagonistic activity. J Pharmacol Exp Ther 274(1):329–336, 1995 7616416
Koro CE, Fedder DO, L’Italien GJ, et al: An assessment of the independent effects of olanzapine and risperidone exposure on the risk of hyperlipidemia in schizophrenic patients. Arch Gen Psychiatry 59(11):1021–1026, 2002a 12418935
Koro CE, Fedder DO, L’Italien GJ, et al: Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study. BMJ 325(7358):243, 2002b 12153919
Leysen JE, Janssen PMF, Schotte A, et al: Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl) 112 (1 suppl):S40–S54, 1993 7530377
Lieberman J, Carson WH, Saha AR, et al: Meta-analysis of the efficacy of aripiprazole in schizophrenia (abstract P.4.E.031). Int J Neuropsychopharmacol 5 (suppl 1): S186, 2002
Maeda K, Lerdrup L, Sugino H, et al: Brexpiprazole II: Antipsychotic-like and precognitive effects of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther 350(3):605–614, 2014a 24947464
Maeda K, Sugino H, Akazawa H, et al: Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther 350(3):589–604, 2014b 24947465
Mallikaarjun S, Kane JM, Bricmont P, et al: Pharmacokinetics, tolerability and safety of aripiprazole once-monthly in adult schizophrenia: an open-label, parallel-arm, multiple-dose study. Schizophr Res 150(1):281–288, 2013 23890595
Marcus RN, McQuade RD, Carson WH, et al: The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 28(2):156–165, 2008 18344725
Marcus RN, Owen R, Kamen L, et al: A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry 48(11):1110–1119, 2009 19797985
Marder SR, McQuade RD, Stock E, et al: Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res 61(2–3):123–136, 2003 12729864
McIntyre RS, McCann SM, Kennedy SH: Antipsychotic metabolic effects: weight gain, diabetes mellitus, and lipid abnormalities. Can J Psychiatry 46(3):273–281, 2001 11320682
McQuade RD, Burris KD, Jordan S, et al: Aripiprazole: a dopamine-serotonin system stabilizer (abstract). Int J Neuropsychopharmacol 5 (suppl 1):S176, 2002
McQuade RD, Stock E, Marcus R, et al: A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double-blind study. J Clin Psychiatry 65 (suppl 18):47–56, 2004 15600384
Meltzer HY: The role of serotonin in antipsychotic drug action. Neuropsychopharmacology 21 (2 suppl):106S–115S, 1999 10432496
Meltzer HY, Risinger R, Nasrallah HA, et al: A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry 76(8):1085–1090, 2015 26114240
Millan MJ: Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther 295(3): 853–861, 2000 11082417
Mintzer JE, Tune LE, Breder CD, et al: Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry 15(11):918–931, 2007 17974864
Nickel MK, Muehlbacher M, Nickel C, et al: Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. Am J Psychiatry 163(5):833–838, 2006 16648324
Nickel MK, Loew TH, Pedrosa Gil F: Aripiprazole in treatment of borderline patients, part II: an 18-month follow-up. Psychopharmacology (Berl) 191(4):1023–1026, 2007 17318503
Otsuka Pharmaceutical: Rexulti: Prescribing information. Tokyo, Japan, Otsuka Pharmaceutical Co, 2015. Available at: https://www.otsuka-us.com/media/images/RexultiPI_544.pdf. Accessed May 24, 2016.
Otsuka Pharmaceutical: Abilify: Prescribing information. Tokyo, Japan, Otsuka Pharmaceutical Co, 2016a. Available at: https://www.otsuka-us.com/media/images/AbilifyPI_538.pdf. Accessed May 24, 2016.
Otsuka Pharmaceutical: Abilify Maintenna: Prescribing information. Tokyo, Japan, Otsuka Pharmaceutical Co, 2016b. Available at: https://www.otsuka-us.com/media/images/AbilifyMPI_539.pdf. Accessed May 24, 2016.
Owen R, Sikich L, Marcus RN, et al: Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics 124(6):1533–1540, 2009 19948625
Petrie JL, Saha AR, McEvoy JP: Aripiprazole, a new novel atypical antipsychotic: phase II clinical trial result (abstract). Eur Neuropsychopharmacol 7 (suppl 2):S227, 1997
Pigott TA, Carson WH, Saha AR, et al; Aripiprazole Study Group: Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clin Psychiatry 64(9):1048–1056, 2003 14628980
Potkin SG, Saha AR, Kujawa MJ, et al: Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 60(7):681–690, 2003 12860772
Pycock CJ, Kerwin RW, Carter CJ: Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature 286(5768):74–76, 1980 7393327
Rao ML, Möller HJ: Biochemical findings of negative symptoms in schizophrenia and their putative relevance to pharmacologic treatment. A review. Neuropsychobiology 30(4):160–172, 1994 7862264
Richelson E: Receptor pharmacology of neuroleptics: relation to clinical effects. J Clin Psychiatry 60 (suppl 10):5–14, 1999 10340682
Sachs G, Sanchez R, Marcus R, et al; Aripiprazole Study Group: Aripiprazole in the treatment of acute manic or mixed episodes in patients with bipolar I disorder: a 3-week placebo-controlled study. J Psychopharmacol 20(4):536–546, 2006 16401666
Sallee FR, Kohegyi E, Zhao J, et al: Children and adolescents with tourette’s disorder: a randomized, double-blind, placebo-controlled trial (abstract). Neuropsychopharmacology 39 (suppl 1): s378–s379, 2014
Seeman P, Niznik HB: Dopamine receptors and transporters in Parkinson’s disease and schizophrenia. FASEB J 4(10):2737–2744, 1990 2197154
Stock E, Marder SR, Saha AR, et al: Safety and tolerability meta-analysis of aripiprazole in schizophrenia (abstract). Int J Neuropsychopharmacol 5 (suppl 1):S185, 2002
Thase ME, Jonas A, Khan A, et al: Aripiprazole monotherapy in nonpsychotic bipolar I depression: results of 2 randomized, placebo-controlled studies. J Clin Psychopharmacol 28(1):13–20, 2008 18204335
Thase ME, Youakim JM, Skuban A, et al: Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry 76(9):1232–1240, 2015a 26301771
Thase ME, Youakim JM, Skuban A, et al: Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry 76(9):1224–1231, 2015b 26301701
Tiihonen J, Kuoppasalmi K, Föhr J, et al: A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry 164(1):160–162, 2007 17202560
Tran-Johnson TK, Sack DA, Marcus RN, et al: Efficacy and safety of intramuscular aripiprazole in patients with acute agitation: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 68(1):111–119, 2007 17284138
Vieta E, Bourin M, Sanchez R, et al; Aripiprazole Study Group: Effectiveness of aripiprazole v. haloperidol in acute bipolar mania: double-blind, randomised, comparative 12-week trial. Br J Psychiatry 187:235–242, 2005 16135860
Vieta E, T’joen C, McQuade RD, et al: Efficacy of adjunctive aripiprazole to either valproate or lithium in bipolar mania patients partially nonresponsive to valproate/lithium monotherapy: a placebo-controlled study. Am J Psychiatry 165(10):1316–1325, 2008 18381903
Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44(7):660–669, 1987 3606332
Yoo HK, Joung YS, Lee JS, et al: A multicenter, randomized, double-blind, placebo-controlled study of aripiprazole in children and adolescents with Tourette’s disorder. J Clin Psychiatry 74(8):e772–e780, 2013 24021518
Zimbroff DL, Marcus RN, Manos G, et al: Management of acute agitation in patients with bipolar disorder: efficacy and safety of intramuscular aripiprazole. J Clin Psychopharmacol 27(2):171–176, 2007 17414241
_____________
This chapter is an update and revision of Sharif ZA, Cole YI, Lieberman JA: “Aripiprazole,” in Essentials of Clinical Psychopharmacology, Third Edition. Edited by Schatzberg AF, Nemeroff CB. Arlington, VA, American Psychiatric Publishing, 2013, pp 291–303.