CHAPTER 30
Ziprasidone
John W. Newcomer, M.D.
Elise Fallucco, M.D.
Martin T. Strassnig, M.D.
History and Discovery
Ziprasidone (CP-88059) is an atypical, or second-generation, antipsychotic agent that has activity for treating positive, negative, cognitive, and affective symptoms of schizophrenia and schizoaffective disorder and for treating mania and mixed states in bipolar disorder, with limited adverse extrapyramidal, sedative, anticholinergic, and cardiometabolic effects. First approved in 2001, this antipsychotic was initially part of a new drug application for the treatment of psychotic disorders submitted to the U.S Food and Drug Administration (FDA) in 1997. Because of concerns regarding an increase in the mean duration of the QT interval, an electrocardiographic measure of the ventricular depolarization and repolarization phases of cardiac conduction, the application was not initially approved. Further studies, designed in collaboration with the FDA, quantified the limited extent of the QTc interval lengthening effect seen with ziprasidone compared with that seen with other agents in wide use; these studies established the safety of ziprasidone with respect to cardiac conduction and a benchmark for the approach to evaluating drug effects on the QT interval that has subsequently been applied to other agents evaluated by the FDA. Ziprasidone has received regulatory approval and is available in more than 92 countries.
Pharmacological Profile
Neuropharmacology and Receptor-Binding Profile
Ziprasidone, or 5-{2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]ethyl}-6-chloro-1,3-dihydro-2H-indol-2-one, is a novel benzisothiazolylpiperazine antipsychotic (Figure 30–1).
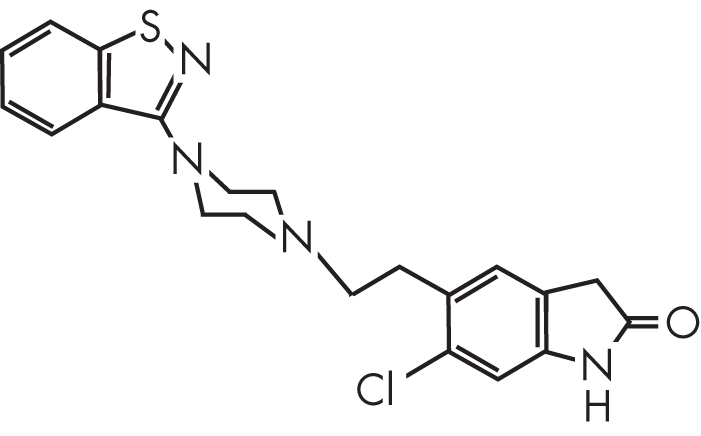
FIGURE 30–1. Chemical structure of ziprasidone.
Dopamine Type 2 and Serotonin Type 2A Receptor Activity
Ziprasidone is a potent antagonist at dopamine type 2 (D2) receptors with inverse agonist activity at serotonin (5-hydroxytryptamine) type 2A (5-HT2A) receptors. D2 receptor antagonism is thought to be a key mechanism explaining efficacy for the treatment of psychotic symptoms (Kapur and Remington 2001); positron emission tomography (PET) studies have shown that clinical antipsychotic response to ziprasidone is predicted by occupancy of at least 60% of striatal D2 receptors. D2 receptor antagonism is also associated with potential liability for extrapyramidal side effects (EPS). However, ziprasidone also has inverse agonist activity at 5-HT2A receptors, an effect that can disinhibit dopamine neurotransmission in the nigrostriatal, mesocortical, and tuberoinfundibular pathways (Kapur and Remington 1996; Schmidt et al. 2001). This effect suggests a mechanism for reduced liability for EPS compared with antipsychotics with unopposed D2 receptor antagonism and may contribute to therapeutic effects. Increased dopamine activity in the prefrontal cortex is linked to efficacy in improving the negative and cognitive symptoms of schizophrenia (Stahl and Shayegan 2003). Enhanced dopaminergic transmission in the tuberoinfundibular pathway minimizes the potential effect of D2 receptor antagonism on prolactin secretion. Ziprasidone’s relatively high in vitro 5-HT2A/D2 receptor affinity ratio, compared with that of other second-generation antipsychotics (SGAs), predicts a low liability for EPS and potential therapeutic benefits for negative symptoms (Altar et al. 1986).
Serotonin Type 1A, 1D, and 2C Receptor Activity
Ziprasidone exhibits antagonist activity at serotonin type 1D (5-HT1D) and type 2C (5-HT2C) receptors and unusual (among SGAs) agonist activity at serotonin type 1A (5-HT1A) receptors (DeLeon et al. 2004; Schmidt et al. 2001). The 5-HT1A affinity is comparable to that of buspirone, an agent with antidepressant and anxiolytic properties (Mazei et al. 2002), suggesting a mechanism that may contribute to beneficial effects on affective, cognitive, and negative symptoms in schizophrenia and schizoaffective disorder (Díaz-Mataix et al. 2005; Ichikawa et al. 2001; Millan 2000; Rollema et al. 2000; Sumiyoshi et al. 2003; Tauscher et al. 2002). Potent antagonism at 5-HT1D receptors has been proposed to potentially mediate antidepressant and anxiolytic effects (Briley and Moret 1993; Zorn et al. 1998). Blockade of 5-HT2C receptors disinhibits both dopamine and norepinephrine neurons in the cortex, an effect that could contribute to improvements in cognitive and affective abnormalities (Bremner et al. 2003; Bymaster et al. 2002; Mazei et al. 2002; Stahl 2003).
Although 5-HT2C receptor antagonist activity might predict weight gain liability, based largely on a 5-HT2C knockout mouse model of obesity (Tecott et al. 1995), clinically significant predictive effects of 5-HT2C receptor antagonist activity on the relative weight gain risk associated with antipsychotic drugs have not been reliably detected (Kroeze et al. 2003), and the weight gain risk associated with ziprasidone is among the lowest of currently available antipsychotics (Allison et al. 1999b).
Serotonin and Norepinephrine Transporter Activity
Another important feature of ziprasidone is its relatively high affinity for serotonin and norepinephrine transporters (Seeger et al. 1995; Tatsumi et al. 1999). In vitro, ziprasidone demonstrates dose-dependent reuptake inhibition of serotonin and norepinephrine transport, with effects ranging up to those of imipramine and amitriptyline (Schmidt et al. 2001), suggesting potential antidepressant activity. In vivo, the clinical significance of ziprasidone’s monoaminergic reuptake inhibition may be limited by plasma protein binding or may be clinically relevant only at daily dosages higher than those currently approved. Monoaminergic reuptake inhibition is associated with hippocampal neurogenesis, suggesting potential value in countering the neuronal cell loss observed in both affective illness and schizophrenia (Arango et al. 2001; Duman 2004; Thome et al. 1998). Relevant to this activity, treatment with ziprasidone or risperidone is associated with an increase in cortical gray matter volume (Garver et al. 2005).
Ziprasidone has a low affinity for histaminergic type 1 (H1), muscarinic type 1 (M1), and α1-noradrenergic receptors. Among the biogenic amine receptors, H1 receptor antagonist activity is the largest predictor of weight gain liability (Kroeze et al. 2003). H1 antagonist activity also predicts sedative effects, which are potentially undesirable for patients aiming to maximize cognitive performance and social, occupational, and community engagement. Low affinity for α1-adrenergic receptors predicts a lower likelihood of orthostatic hypotension and sedation with ziprasidone than with commonly used antipsychotics with potent α1-adrenergic receptor antagonist activity. Low affinity for M1 receptors predicts a low risk for anticholinergic side effects such as dry mouth, blurry vision, urinary retention, constipation, confusion, and memory impairment.
Ziprasidone’s complex neuropharmacology provides explanatory support for observed treatment effects on psychotic and affective symptoms of schizophrenia, schizoaffective disorder, and bipolar disorder and for the observed favorable tolerability profile with minimal EPS and minimal metabolic side effects (Stahl and Shayegan 2003).
Positron Emission Tomography Studies
An in vivo PET study (Mamo et al. 2004) examining the affinity of ziprasidone for D2 and 5-HT2 receptors observed that optimal D2 receptor occupancy occurs at the high end of ziprasidone’s initially recommended dosage range. In this study, the ziprasidone plasma concentration associated with 50% of maximal D2 receptor occupancy was more than twice the plasma concentration associated with 50% of maximal 5-HT2 receptor occupancy. Using an imaging protocol where 60% or greater dopamine D2 receptor occupancy is generally predictive of antipsychotic activity, approximately 60% D2 occupancy was observed in relation to plasma concentrations equivalent to those attained with a dosage at or above 120 mg/day. These results, consistent with clinical trial results discussed later in this chapter (see “Indications and Efficacy”), suggest that antipsychotic activity with ziprasidone is most commonly associated with dosages of 120 mg/day or greater (Figure 30–2).
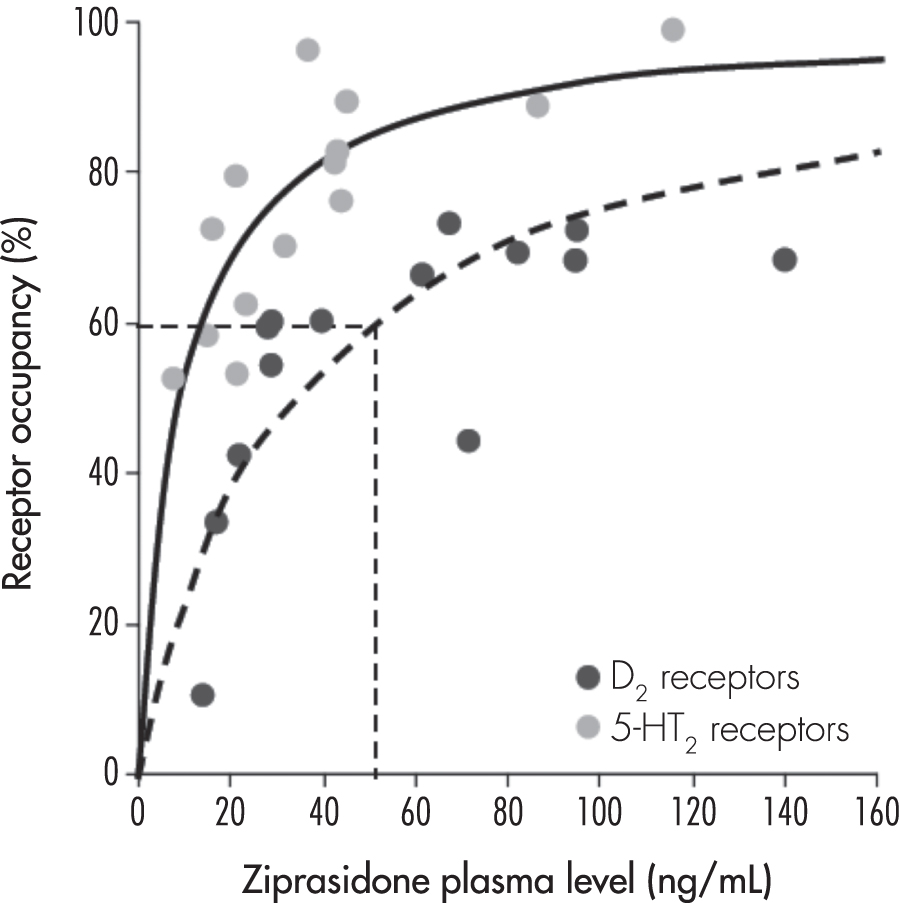
FIGURE 30–2. Relationship between dopamine2 (D2) and serotonin2 (5-HT2) receptor occupancy and ziprasidone plasma levels in 16 patients with schizophrenia or schizoaffective disorder receiving therapeutic dosages of ziprasidone.
Dotted straight lines represent the minimum D2 receptor occupancy and plasma concentration that would be expected to be associated with a clinical antipsychotic response, corresponding to a ziprasidone dosage of approximately 120 mg/day.
Source. Adapted from Mamo et al. 2004.
Dosing Recommendations
In addition to the PET data just described, evidence from clinical trials suggests that ziprasidone target dosages should be higher than those originally recommended. In the United States, it was initially recommended that ziprasidone treatment in patients with schizophrenia be initiated at a dosage of 20 mg twice daily, with the dosage then titrated at intervals of no less than 2 days to a maximum of 80 mg twice daily (Pfizer Inc. 2008). In contrast, subsequent FDA approval of ziprasidone for the treatment of bipolar mania included a recommendation that treatment be initiated at 40 mg twice daily with a more rapid titration; on the second day of treatment, the dosage might be increased to 60 or 80 mg twice daily, with subsequent adjustment based on tolerability and efficacy within a 40- to 80-mg twice-daily range.
Ziprasidone dosages of 120–160 mg/day are observed to be more effective than lower dosages in the treatment of acute schizophrenia (Kane 2003b) and bipolar disorders (Citrome et al. 2009b) in adults and also are associated with lower rates of medication discontinuation (Citrome et al. 2009c). A 6-month prospective, observational, naturalistic, uncontrolled study in Spain observed that dosages greater than 120 mg/day were associated with a lower risk of discontinuation for any cause (Arango et al. 2007). In an analysis of commercial and Medicare prescription databases, Citrome et al. (2009a) observed significantly lower discontinuation rates among schizophrenia and bipolar disorder patients receiving ziprasidone at dosages of 120–160 mg/day compared with those receiving ziprasidone at lower dosages. Similarly, a European observational multicenter trial found that initial and overall underdosing of ziprasidone were associated with high discontinuation rates (Kudla et al. 2007), and a pooled analysis of both flexible-dose and fixed-dose studies (N=2,174) observed greater efficacy in patients who received an initial dosage of 80 mg/day compared with patients who received an initial dosage of 40 mg/day (Murray et al. 2004). Finally, two large observational database analyses suggested that higher dosages of ziprasidone are associated with better treatment outcomes than lower dosages (Joyce et al. 2006; Mullins et al. 2006). Both studies used prescription refills as an indicator of prescription adherence. Joyce et al. (2006) examined records from more than 1,000 commercially insured patients with schizophrenia or schizoaffective disorder and concluded that an initial daily dosage of 120–160 mg was associated with a significantly lower risk of medication discontinuation at 6 months than an initial daily dosage of 60–80 mg. Mullins et al. (2006) evaluated more than 1,000 Medicaid recipients with schizophrenia and similarly concluded that patients receiving an initial dosage of 120–160 mg/day had lower rates of medication discontinuation than patients receiving 20–60 mg/day. Reported clinical experience with ziprasidone has also suggested the need for dosages greater than 160 mg/day in selected patients (Citrome et al. 2009a; Harvey and Bowie 2005; Nemeroff et al. 2005). A dosage of 320 mg/day, twice the maximum recommended dosage of 160 mg/day, did not lead to any additional symptom improvement in a small 8-week placebo-controlled trial as compared with a dosage of 160 mg/day, and plasma drug levels were similar between the two dosages at the end of the study period. There was a trend toward increasing diastolic blood pressure, more prominent negative symptoms, and greater QTc prolongation (Goff et al. 2013) with the 320 mg/day dosage.
Taken together, results from receptor occupancy studies, clinical trials, and pharmacoepidemiological analyses support the conclusion that initiation and treatment with ziprasidone at dosages greater than 120 mg/day, with rapid titration, are more likely to be effective than lower dosages in the treatment of schizophrenia, schizoaffective disorder, and bipolar disorder, while excessive dosages may incur additional side effects rather than additional efficacy.
Pharmacokinetics and Disposition
Absorption and Distribution
On the basis of evidence of enhanced absorption of ziprasidone in the presence of food, it is recommended that oral ziprasidone be taken with meals of at least 500 kcal to avoid substantial reduction in drug absorption (Gandelman et al. 2009) that cannot be effectively compensated for by increasing the dosage (Citrome 2009). Administration with food increases absorption by more than 50%, giving ziprasidone an oral bioavailability of approximately 60% (Pfizer Inc. 2008). Maximal plasma concentration (Cmax) is achieved within 3.7–4.7 hours and reaches 45–139 μg/L in healthy volunteers receiving 20–60 mg twice daily, and steady-state serum concentrations occur within 1–3 days of twice-daily dosing (Hamelin et al. 1998; Miceli et al. 2000c). In contrast to oral administration, intramuscular administration of ziprasidone results in 100% bioavailability. A therapeutic plasma level is reached within 10 minutes, and Cmax is achieved within 30 minutes of administration of a 20-mg dose (Pfizer Inc. 2008).
The mean apparent volume of distribution of ziprasidone is 1.5 L/kg (Pfizer Inc. 2008), which is lower than that of many other antipsychotic drugs. Given the wider potential for unwanted interactions with various intracellular targets that has been observed with lipophilic drugs having a high volume of distribution (Dwyer et al. 1999), this may be a favorable attribute for ziprasidone and other similar compounds. Ziprasidone is more than 99% bound to plasma proteins.
Metabolism and Elimination
Ziprasidone is extensively metabolized, with a mean terminal elimination half-life of approximately 7 hours after oral administration within the recommended clinical dosage range (Pfizer Inc. 2008). The elimination half-life of intramuscular ziprasidone is less than 3 hours with a single dose (Brook et al. 2000). Ziprasidone is cleared primarily via three metabolic pathways to yield four major circulating metabolites. Elimination occurs primarily through hepatic metabolism, with less than one-third of metabolic clearance mediated via cytochrome P450 (CYP)–catalyzed oxidation and approximately two-thirds via reduction of the parent compound by aldehyde oxidase to dihydroziprasidone, which then undergoes S-methylation. The literature reports no commonly encountered clinically significant pharmacological inhibitors of aldehyde oxidase, suggesting limited real-world potential for drug–drug interactions that would alter the clinical activity of ziprasidone (Obach et al. 2004).
Additional secondary metabolic pathways include N-dealkylation (via CYP enzymes 3A4 and 1A2) and direct S-oxidation (via CYP3A4) (Beedham et al. 2003; Prakash et al. 2000). S-Methyl-dihydroziprasidone is the only active metabolite, with lower D2 receptor affinity and no significant binding to H1, M1, or α1- and α2-adrenergic receptors. A small amount of the parent compound is excreted unchanged in the urine (<1%) and feces (<4%).
There are no clinically significant age- or sex-related differences in the pharmacokinetics of oral ziprasidone (Pfizer Inc. 2008). Hepatic impairment might be expected to increase the area under the time–concentration curve (AUC). A multiple-dose study (Everson et al. 2000) comparing subjects with clinically significant (Child-Pugh Class A and B) cirrhosis versus healthy control subjects indicated that 12 hours after administration of ziprasidone, the AUC was 13% and 34% greater in subjects with Child-Pugh Class A and B cirrhosis, respectively, than in matched control subjects, suggesting that dose adjustments are generally not mandatory for patients with hepatic impairment. Impairment in renal function is unlikely to significantly alter the pharmacokinetics of oral ziprasidone, suggesting that ziprasidone would not be removed by hemodialysis (Pfizer Inc. 2008). Intramuscular ziprasidone has not been systematically evaluated in the elderly or in patients with hepatic or renal impairment. Intramuscular ziprasidone contains a cyclodextrin excipient that is cleared by renal filtration; thus, it should be administered with caution to patients with impaired renal function (Pfizer Inc. 2008).
Impact of Food on Pharmacokinetics
Pharmacokinetic studies have examined ziprasidone bioavailability under fasting conditions and after eating food with varying caloric and fat composition to better understand effects of food intake on drug availability (Gandelman et al. 2009; Lombardo et al. 2007). In an open-label, nonrandomized six-way crossover study, healthy adults received single doses of ziprasidone under fasting conditions and then under fed conditions with a standard meal of 800–1,000 calories. Dose-proportional increases in ziprasidone AUC and Cmax were observed under fed but not fasting conditions. Cmax was significantly higher in fed states than in fasting states at doses of 40 mg (63% higher) and 80 mg (97% higher). Results from two additional open-label crossover studies further clarified the factors regulating drug bioavailability (Gandelman et al. 2009; Lombardo et al. 2007) and indicated that 1) medium-calorie meals (500 calories) are associated with ziprasidone exposure close to the exposure that can be achieved with high-calorie meals (1,000 calories) and nearly twice the exposure observed under fasting conditions, and 2) absorption is not significantly influenced by the fat content of the meal. These studies suggest that the administration of ziprasidone with even a low-fat meal of at least 500 calories provides linear pharmacokinetics and optimal absorption. In addition, the results suggest that larger meal bulk, sufficient to slow gastric and duodenal transit time (e.g., a bowl of oatmeal), rather than fat content or specific calorie counts, may be an important contributor to reliable dose-dependent drug absorption with meals.
Indications and Efficacy
Schizophrenia and Schizoaffective Disorder
Acute Treatment
Ziprasidone is indicated for the acute treatment of schizophrenia and schizoaffective disorder. Its efficacy in the treatment of hospitalized patients with acute schizophrenia or schizoaffective disorder has been demonstrated in a series of double-blind, placebo-controlled trials of 4–6 weeks’ duration (Daniel et al. 1999; Kane 2003b; Keck et al. 1998, 2001). Additional short-term (4- to 8-week) randomized, double-blind treatment studies using active antipsychotic comparator agents have indicated that ziprasidone has efficacy comparable to that of haloperidol, risperidone, and olanzapine for the treatment of positive symptoms and overall psychopathology (Addington et al. 2004; Goff et al. 1998; Simpson et al. 2004a). In a pooled analysis of four short-term placebo-controlled trials and three active-comparator trials, Murray et al. (2004) demonstrated that ziprasidone dosages of at least 120 mg/day, in comparison with lower dosages, are associated with a more rapid and favorable response in overall psychopathology as well as a lower discontinuation rate due to inadequate clinical response, suggesting the importance of rapid titration to at least 120 mg/day (Kane 2003b; McCue et al. 2006).
Suboptimal dosing, inadequate titration regimens, and failure to administer ziprasidone with food may have negatively affected its performance in some clinical trials. Some clinical trials included efficacy analyses on dosages that would now be considered suboptimal (i.e., <120 mg/day), whereas other studies used prolonged titration of ziprasidone, with a therapeutic dosage not reached until 1 week or more into the study. Clinical trials conducted before the release of pharmacokinetic data by Lombardo et al. (2007) may not have been designed to ensure that ziprasidone was administered with food for optimal oral absorption.
Ziprasidone has also been studied in the treatment of early psychosis and schizophrenia. In a study by Johnsen et al. (2010), patients admitted to the emergency ward with early psychosis were consecutively randomly assigned to receive one of several different SGAs: ziprasidone, olanzapine, quetiapine, or risperidone. No clinically significant differences in effectiveness of the tested antipsychotics emerged after a 2-year follow-up period. Crespo-Facorro et al. (2013, 2014), in a 12-week prospective open-label, randomized trial followed by a year-long extension period comparing quetiapine, aripiprazole, and ziprasidone for the treatment of first-episode psychosis, found significantly greater discontinuation rates with quetiapine than with aripiprazole or ziprasidone, owing to “insufficient” efficacy of quetiapine and comparable efficacy of the other two tested agents.
A series of meta-analyses found no consistent differences among the SGAs, either when comparing agents within the same class or when comparing SGAs with first-generation antipsychotics (FGAs) (Bagnall et al. 2003; Geddes et al. 2000; Leucht et al. 1999; Srisurapanont and Maneeton 1999; Tandon and Fleischhacker 2005). One meta-analysis of randomized controlled trials (RCTs) by Davis et al. (2003) suggested that although some SGAs (i.e., clozapine, risperidone, olanzapine, and amisulpride) were significantly more efficacious than FGAs, ziprasidone was not. It is important to note that this meta-analysis excluded data relating to low dosages of other antipsychotics (olanzapine <11 mg/day and risperidone <4 mg/day) but included data on ziprasidone dosages as low as 80 mg/day. In addition, the Davis et al. meta-analysis included relatively few studies of ziprasidone (4 studies as compared with 31 studies of clozapine, 22 studies of risperidone, and 14 studies of olanzapine), leaving significance testing for this agent more vulnerable to issues associated with individual studies. Leucht et al. (2013) used a novel approach, a Bayesian-framework, multiple-treatment meta-analysis of available RCT data, comparing 15 antipsychotic drugs and placebo in the acute treatment of schizophrenia, and concluded that antipsychotics differed substantially in side effects and that, while all antipsychotics were better than placebo, small but robust differences exist in efficacy, with clozapine representing the most effective—but not necessarily the best tolerated—antipsychotic.
Using Medicaid claims data, Olfson et al. (2012) compared the effectiveness (as measured by rates of medication discontinuation and hospital admission) of commonly prescribed SGA medications in child and adolescent outpatients (ages 6–17 years) with schizophrenia or related disorders. Most youth with early psychosis (defined as onset before age 18 years) treated with quetiapine (70.7%), ziprasidone (73.3%), olanzapine (73.1%), risperidone (74.4%), or aripiprazole (76.5%) discontinued the medication within the first 180 days following medication initiation. Studies like these underscore both the comparable effectiveness among SGA agents and the high discontinuation rates associated with available treatments, the latter in part related to unwanted medication-induced adverse events. The favorable side-effect profile of ziprasidone recommends it as an important first-line option in the treatment of early psychosis.
Maintenance Therapy
The maintenance efficacy of ziprasidone in treating schizophrenia and schizoaffective disorder has been studied in a series of double-blind and open-label extension trials (Arato et al. 2002; Hirsch et al. 2002; Kane 2003a; Schooler 2003; Simpson et al. 2002, 2004b). These studies indicate that long-term therapy with ziprasidone maintains clinical response and is effective in preventing relapse.
Maintenance of effect. In two maintenance-of-effect studies, patients with schizophrenia or schizoaffective disorder who had previously demonstrated acute response to treatment (defined as a ≥20% decrease in Positive and Negative Syndrome Scale [PANSS]–Total score and a Clinical Global Impression [CGI] scale score of ≤2) were randomly assigned to receive either ziprasidone or a comparator antipsychotic agent for at least 26 weeks (Addington et al. 2009; Schooler 2003; Simpson et al. 2002, 2005). In both of these studies, the ziprasidone treatment groups demonstrated significant improvements from baseline in overall psychopathology, as measured by mean changes in symptom ratings using PANSS–Total, PANSS–Negative subscale, Brief Psychiatric Rating Scale–Depression Factor (BPRSd), and CGI–Severity (CGI-S) scores. These improvements were comparable to those seen in the olanzapine (Simpson et al. 2005) and risperidone (Addington et al. 2009) treatment groups.
Relapse prevention. To evaluate the efficacy of ziprasidone for relapse prevention, the Ziprasidone Extended Use in Schizophrenia (ZEUS) study enrolled stable inpatients with chronic schizophrenia and randomly assigned participants to 1 year of treatment with ziprasidone 40 mg/day (n=72), 80 mg/day (n=68), or 160 mg/day (n=67) or placebo (n=71), with a planned primary Kaplan-Meier analysis of time to relapse (Arato et al. 2002). In this study, all three dosages of ziprasidone were superior to placebo in the prevention of relapse. In addition, a penultimate-observation-carried-forward analysis (in which the last visit prior to relapse is excluded) was performed to filter out clinical worsening associated with relapse that might otherwise obscure symptom response trends during the rest of maintenance therapy (O’Connor and Schooler 2003). This analysis indicated that nonrelapsing patients treated with ziprasidone experienced modest symptomatic improvement during maintenance treatment. This study, like a number of other studies of other antipsychotic agents in schizophrenia patients, was limited by the relatively high level of attrition observed in all groups over the year of treatment.
Long-term response to treatment in symptomatic patients. A number of long-term double-blind trials designed to examine the efficacy of ziprasidone in symptomatic patients with schizophrenia have been performed (Breier et al. 2005; Hirsch et al. 2002; Kinon et al. 2006; Simpson et al. 2004a, 2005). Hirsch et al. (2002) compared the efficacy of ziprasidone (n=148) and haloperidol (n=153) in a 28-week double-blind trial of stable outpatients with schizophrenia with prominent negative symptoms. In this study, ziprasidone and haloperidol were similarly efficacious in reducing overall psychopathology, with an advantage for ziprasidone in the percentage of patients classified as negative symptom responders. Breier et al. (2005) conducted a 28-week study of ziprasidone and olanzapine in outpatients as well as inpatients (N=548) with active symptoms. In this study, olanzapine treatment was associated with greater improvement from baseline in total psychopathology scores (PANSS–Total, the primary efficacy measure) compared with ziprasidone and a higher rate of criterion-level response to treatment. Simpson et al. (2004a) conducted a 6-week double-blind, parallel-design flexible-dose comparison (N=269) of ziprasidone (n=136) and olanzapine (n=133) in which patients who showed at least minimal response (CGI–Improvement [CGI-I] score ≤2 or ≥20% reduction in PANSS–Total score) were enrolled in a 6-month double-blind continuation trial (Simpson et al. 2005). In both the 6-week and the 6-month analyses, no differences between the treatment groups were detected on any primary (e.g., Brief Psychiatric Rating Scale [BPRS] total score, CGI-S) or secondary (e.g., PANSS–Total, CGI-I) measures.
Potkin et al. (2009) compared the effects of ziprasidone and haloperidol in a 196-week double-blind study using the Andreasen criteria for remission (Andreasen et al. 2005) by longitudinal analysis. During the initial 40-week study, no differences in PANSS or Global Assessment of Functioning Scale (GAF) score changes between the two antipsychotics compared emerged. In a subsequent 3-year extension study, ziprasidone-treated subjects were more likely to achieve remission (51%) compared with haloperidol-treated subjects (40%). The PANSS–Total and GAF score trajectories favored the higher 80–160 mg/day ziprasidone dosage as opposed to lower dosages, and the superior outcome for ziprasidone versus haloperidol may have been due in part to tolerability and adherence advantages.
Clinical effectiveness. There have been a number of effectiveness trials involving ziprasidone and using various definitions for effectiveness. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) studies, funded by the National Institute of Mental Health (NIMH), included a long-term, double-blind, randomized study of patients with schizophrenia (N=1,493; Lieberman et al. 2005). Phase 1 of the CATIE schizophrenia study compared ziprasidone, olanzapine, quetiapine, risperidone, and perphenazine on a primary endpoint of time to discontinuation for lack of efficacy or for any cause. Because of the timing of its FDA approval, ziprasidone was added to the study after enrollment had begun for all other treatment arms; this resulted in a smaller sample size for the ziprasidone treatment arm and some limitations regarding conclusions about ziprasidone in phase 1 of the study.
The primary analysis for the phase 1 study detected significant differences in time to discontinuation across the treatment groups overall. The longest time to discontinuation was in the olanzapine group. In the total study sample, no significant differences were seen in the time to discontinuation between the ziprasidone and olanzapine treatment groups, or between the ziprasidone group and other antipsychotic treatment groups.
Several considerations in this complex study are worth mentioning. Very few patients who entered the CATIE schizophrenia study were currently (i.e., prior to study entry) taking the relatively newly available medication, ziprasidone, compared with the number of patients taking the other antipsychotic medications in the trial. This resulted in a larger proportion of patients assigned to the ziprasidone treatment arm who were just starting to take a new medication and discontinuing their prior treatment. For example, 23% of subjects randomly assigned to receive olanzapine treatment were already receiving olanzapine monotherapy as their ongoing treatment, requiring no medication discontinuation or new drug initiation. Supplemental analysis of phase 1 CATIE data by Essock et al. (2006) indicated the overall importance, in terms of subsequent discontinuation rates, of whether randomized subjects were switching medications or whether the study randomization allowed them to continue receiving their prior treatment. A significantly higher rate of subsequent discontinuation was observed in patients who actually made a medication switch compared with those who were randomly assigned to stay with the same medication they had been taking prior to the trial. This effect of switching medications therefore favored treatment arms with a larger percentage of “nonswitchers” (i.e., olanzapine and risperidone recipients in the CATIE phase 1 study). In a reanalysis of phase 1 CATIE data that excluded those patients randomly assigned to continue taking the antipsychotic that they were already taking at baseline, Essock et al. (2006) found that differences between rates of discontinuation in the ziprasidone group and rates in the other antipsychotic groups were attenuated, and no statistically significant differences in the primary outcome measure were observed for any agent.
The Ziprasidone Experience in Schizophrenia in Germany/Austria (ZEISIG) study investigated the effectiveness of ziprasidone as measured by discontinuation rates and mean changes on the BPRS Total score in moderately ill and reasonably stable patients (N=276) with schizophrenia or schizoaffective disorder (Kudla et al. 2007). Approximately 60% of subjects discontinued ziprasidone prematurely, most within the first 4 weeks of study treatment. Among study completers, ziprasidone was associated with significant improvements in BPRS total score. The relatively high rate of discontinuation may be explained in part by the planned dosing strategy. In this study, ziprasidone use was initiated at a low dosage of 40 mg/day, which is now known to be associated with higher discontinuation rates and shorter durations of therapy compared with higher dosages (Joyce et al. 2006; Mullins et al. 2006). The maximal dosage allowed was 160 mg/day, which may be insufficient for some patients (Harvey and Bowie 2005; Nemeroff et al. 2005).
Arango et al. (2007), of the Ziprasidone in Spain Study Group, examined the effectiveness of ziprasidone (N=1,022 in the primary analysis sample) as measured by response rate (defined as a ≥30% reduction in the PANSS–Total score). Nearly half of the patients experienced the defined level of clinical response, and patients overall had significant and clinically relevant mean reductions in both the PANSS–Total score and the Positive, Negative, and General Psychopathology subscale scores (effect sizes were 1.60, 1.83, 0.62, and 1.40, respectively). Compared with lower dosages, ziprasidone dosages greater than 120 mg/day were associated with a lower risk of discontinuation for any cause.
Díaz-Marsá et al. (2009) conducted a prospective, uncontrolled, naturalistic study to evaluate the effectiveness and tolerability of oral ziprasidone in psychiatric inpatients with an acute exacerbation of schizophrenia or schizoaffective disorder. Among the 196 patients enrolled, the mean dosage of ziprasidone at discharge was 186.3±67.7 mg/day. Progressive and statistically significant improvements in BPRS- and CGI-measured symptom severity were observed from the first week through discharge (after 23.4±34.2 days). Changes from baseline to study endpoint were deemed clinically relevant, with reported effect sizes greater than d=0.8, indicating that the results are of notable clinical—not just statistical—significance.
The European First Episode Schizophrenia Trial (EUFEST; Kahn et al. 2008) included 49 sites in Europe and Israel and assessed 498 first-episode patients ages 18–40 years who had experienced psychosis for less than 2 years, had been exposed to antipsychotic drugs for less than 2 weeks during the preceding year, and had less than 6 weeks of total lifetime exposure to antipsychotic drugs. Participants were randomly assigned to receive haloperidol (which served as the FGA comparator) 1–4 mg/day, amisulpride 200–800 mg/day, olanzapine 5–20 mg/day, quetiapine 200–750 mg/day, or ziprasidone 40–160 mg/day and were followed up for 1 year. Analysis of all-cause treatment discontinuation revealed rates of 40% for haloperidol, 40% for amisulpride, 33% for olanzapine, 53% for quetiapine, and 45% for ziprasidone. Symptom reductions were similar in all groups (approximately 60%). In addition to efficacy considerations, tolerability differences—whether due to different pharmacological profiles or to patient preferences—should guide the clinician in choosing an appropriate first-line treatment.
Efficacy by Symptom Type
Efficacy for cognitive symptoms of schizophrenia. The effect of ziprasidone on cognitive function in schizophrenia patients was evaluated by a battery of cognitive tests included in a double-blind olanzapine comparator study that evaluated changes at 6 weeks and 6 months (Harvey et al. 2004, 2006a). Antipsychotic treatment with ziprasidone and with olanzapine both resulted in significant cognitive improvements from baseline in attention memory, working memory, motor speed, and executive functions, with olanzapine also associated with improvement in verbal fluency. Further improvements in both treatment groups were observed from the end of 6 weeks to the 6-month assessment time point on verbal learning, executive functioning, and verbal fluency, with no differences between treatment groups. It should be noted that despite these improvements, a substantial proportion of patients studied continued to experience clinically significant cognitive impairment posttreatment. Neuropsychological improvements in general are not related to clinical changes (Harvey et al. 2006b).
Data from the CATIE schizophrenia study indicate that treatment with all of the antipsychotics tested (i.e., ziprasidone, perphenazine, olanzapine, risperidone, and quetiapine) was associated with a small but significant improvement in cognition after 2 months of treatment, with no significant difference between ziprasidone and the other antipsychotics (Keefe et al. 2007). Cognitive improvement predicted a longer time to treatment discontinuation, independent of symptom improvement, in patients treated with quetiapine or ziprasidone.
Efficacy for affective symptoms. Ziprasidone has been hypothesized to be a promising treatment for mood disorders, based on its unique in vitro potency as a serotonin and norepinephrine reuptake inhibitor comparable to that of known antidepressants (see “Neuropharmacology and Receptor-Binding Profile” section earlier in this chapter). Addressing the question of ziprasidone’s potential antidepressant efficacy in schizophrenia patients with comorbid affective symptoms, data can be examined from randomized, double-blind, placebo-controlled clinical trials (Daniel et al. 1999; Keck et al. 1998, 2001) and from double-blind head-to-head trials comparing ziprasidone with risperidone or olanzapine (Kane 2003b). The results of placebo-controlled studies (Daniel et al. 1999; Keck et al. 1998) suggest that treatment of schizophrenia and schizoaffective disorder with ziprasidone is associated with significant improvement in comorbid depressive symptoms, based on intent-to-treat analyses, but sometimes only in the subset of patients with higher levels of baseline depression. The baseline severity of depressive symptoms in these studies tends to be relatively mild, so subgroups of patients with more pronounced comorbid depressive symptoms at baseline were also analyzed; the antidepressant effect of ziprasidone was larger than that of placebo in these analyses. In the two active-comparator studies, improvement in depression and anxiety symptoms in patients receiving ziprasidone was comparable to the improvement in olanzapine recipients but greater than the improvement in risperidone recipients. A smaller study (Kinon et al. 2006) compared the efficacy of olanzapine and ziprasidone over 24 weeks in the treatment of schizophrenia or schizoaffective disorder patients with prominent depressive symptoms. Both treatment groups had significant improvements in depressive symptoms for the first 8 weeks, with olanzapine-treated patients showing significantly greater improvements in depressive symptoms at study endpoint. However, interpretation of the findings from this study is limited by that fact that a substantial number of patients (52.8% of N=394 at study entry) received concurrent treatment with nonstandardized antidepressants. These overall results provide preliminary evidence suggesting that ziprasidone, like some other antipsychotic agents, may be effective in treating comorbid depressive symptoms in patients with schizophrenia and schizoaffective disorder.
Efficacy for social deficits and improvement in quality of life. The NIMH CATIE study is the largest trial to date to have examined the effect of ziprasidone and other antipsychotics on psychosocial functioning in patients with schizophrenia (Swartz et al. 2007). This study employed the Quality of Life Scale, a widely used clinician-rated measurement (Heinrichs et al. 1984), to assess changes in social functioning, interpersonal relationships, vocational functioning, and psychological well-being. One-third of the patients in the phase 1 study antipsychotic treatment groups made modest improvements on the Quality of Life Scale from baseline to the 12-month endpoint (average effect size, 0.19 standard deviation units), with no significant differences between the agents.
The effect of ziprasidone on social functioning has also been evaluated using the Prosocial subscale of the PANSS, including items related to active and passive social avoidance, emotional withdrawal, stereotypical thinking, and suspiciousness (Purnine et al. 2000). In three separate but related studies from one group, stable patients taking FGAs, olanzapine, or risperidone were switched to ziprasidone and followed for 6 weeks with ratings of safety, efficacy, and effectiveness (Weiden et al. 2003b). Six weeks of treatment with ziprasidone in all three prior-treatment groups resulted in significant improvement on the PANSS Prosocial subscale (Loebel et al. 2004). The interpretation of results as being specific to ziprasidone use, rather than being simply an effect of extended, closely monitored treatment, is complicated by the absence of a control other than pretreatment baseline ratings.
Harvey et al. (2009) examined quality-of-life changes in community-dwelling patients with schizophrenia randomly assigned to receive either haloperidol (n=47) or ziprasidone (n=139) over a follow-up interval of up to 196 weeks. Long-term treatment with ziprasidone was associated with greater functional gains than treatment with haloperidol. Both treatment retention and functional gains favored ziprasidone in this long-term study, suggesting superior efficacy and tolerability, which may reflect back favorably on adherence rates. Post hoc analysis revealed the most significant quality-of-life improvements in the high-dosage ziprasidone group (80–160 mg/day) (Stahl et al. 2010b), concordant with recent recommendations that higher dosages of ziprasidone may be more effective than lower dosages.
Treatment-Resistant Schizophrenia
Several studies have evaluated ziprasidone use in refractory schizophrenia, albeit using different criteria for “refractory.” A 12-week double-blind comparison of ziprasidone and chlorpromazine (N=306 patients) defined treatment-resistant status as inability to achieve criterion-level response after 6 weeks of prospective treatment with haloperidol (Kane et al. 2006). The mean daily dose of ziprasidone at study endpoint was approximately 154 mg, compared with a mean daily chlorpromazine dose of approximately 744 mg. Treatment with ziprasidone produced significantly greater improvement at endpoint in PANSS–Negative subscale scores compared with chlorpromazine. In addition, ziprasidone treatment was associated with a 1.3-fold higher likelihood of achieving a 50% reduction in BPRS total score compared with chlorpromazine treatment. The Monitoring Oral Ziprasidone As Rescue Therapy (MOZART) study in antipsychotic-resistant/intolerant patients (Sacchetti et al. 2009), an 18-week randomized, flexible-dose, double-blind trial, evaluated ziprasidone as an alternative to clozapine in treatment-refractory schizophrenia. Patients had a history of nonresponse and/or intolerance to at least three acute cycles of different antipsychotic medications given at therapeutic doses, with persistent PANSS–Total scores of at least 80. Patients were randomly assigned to receive ziprasidone 80–160 mg/day or clozapine 250–600 mg/day. A progressive and significant reduction from baseline in PANSS–Total score was observed from day 11 in both study arms, without between-drug differences and with similar rates of early discontinuation due to adverse events. Ziprasidone had a more tolerable metabolic profile in the short-term treatment.
A small amount of literature suggests that the addition of a second antipsychotic medication to clozapine in patients who do not respond to or cannot tolerate standard dosages of clozapine may provide additional benefits. In the context of safety concerns and increasing health care costs, there is currently limited empirical evidence for the efficacy and safety of such antipsychotic combinations (Kreyenbuhl et al. 2007). However, adjunctive treatment with ziprasidone or risperidone, for example, was found helpful in patients with refractory schizophrenia that was incompletely responsive to clozapine (Zink et al. 2009). Both adjunctive antipsychotics produced additional reductions on PANSS–Positive and –Negative symptom subscale scores after 6 weeks of treatment, and the intervention was well tolerated. Further investigations are needed before definitive recommendations can be made, and treatment resistance should be operationalized uniformly so as to facilitate comparative research.
Switching From Other Antipsychotics
The efficacy of ziprasidone has been found to be comparable to that of other SGAs and FGAs during both acute and maintenance treatment of schizophrenia and schizoaffective disorder. Evidence also points to the safety—particularly the cardiometabolic safety—of ziprasidone compared with other antipsychotics (see “Side Effects and Toxicology” section later in this chapter). These results support interest in switching from antipsychotic treatment with other agents to treatment with ziprasidone.
Several open-label medication-switching studies evaluated strategies for switching from other antipsychotics to ziprasidone on measures of efficacy, safety, and tolerability, including the effect of different titration schedules on the outcome (Weiden et al. 2003b). In each study, patients were randomly assigned to one of three switching strategies to be completed in 1 week: 1) immediately discontinuing the previous antipsychotic and immediately starting ziprasidone the next day; 2) lowering the dose of the previous antipsychotic by half while simultaneously starting ziprasidone; or 3) overlapping the start of ziprasidone with the full dosage of the prior antipsychotic and then gradually reducing the prior antipsychotic dosage after 4 days of ziprasidone therapy.
For these switching strategies, the starting dosage of ziprasidone was 80 mg/day (40 mg twice daily), with subsequent dosage adjustments based on clinical judgment. In one study, patients taking high-potency FGAs such as haloperidol (N=108) were switched to ziprasidone. In the second study, patients (N=58) were switched from risperidone to ziprasidone. In the third study, patients (N=104) were switched from olanzapine to ziprasidone. Discontinuation rates were low in all three studies, ranging from 2%–6% for lack of efficacy to 6%–9% for adverse events. Among study completers, statistically significant improvements were observed in PANSS–Total, PANSS–Positive, and PANSS–Negative subscale scores and BPRS total scores. Different switching strategies were not associated with a different likelihood of trial completion or different magnitude of clinical response.
Rossi et al. (2011) examined pooled data from 10 previously completed “switch” studies involving a total of 1,395 patients who were switched to ziprasidone. Switching from FGAs or other SGAs generally resulted in maintenance or improvement of efficacy across all studied symptom domains, including improvements in tolerability and acute and long-term benefits regarding cardiometabolic parameters. The recommended titration schedule was a “plateau cross-titration strategy,” which the authors described as rapid uptitration of ziprasidone to a dosage range of 60–80 mg administered twice daily with food. To facilitate the crossover and minimize patient discomfort, temporary coadministration of benzodiazepines, anticholinergics, or beta-blockers was recommended for management of potential rebound effects due to differences in pharmacological profiles of the pre-switch medications and ziprasidone.
The balance of the evidence from studies examining strategies for switching to ziprasidone favors rapid uptitration of ziprasidone to a comparatively higher dosage (up to 160 mg/day) combined with rapid discontinuation of the previous agent, including management of specific rebound effects combined with close clinical monitoring during the switch. Predictable changes in tolerability (e.g., weight loss when switched from olanzapine, reduction in EPS when switched from haloperidol) are observed along with maintenance of efficacy.
Bipolar Disorder
Acute Mania
Ziprasidone has received regulatory approval (e.g., by the FDA) for the acute treatment of bipolar mania, with efficacy for acute mania demonstrated in two double-blind, placebo-controlled trials, each 3 weeks in duration, in patients with bipolar I disorder (Keck et al. 2003b; Potkin et al. 2005). In both studies, onset of action was rapid (within 48 hours) and sustained through 3 weeks of treatment in patients with bipolar mania or bipolar mixed states, with or without psychotic symptoms. (The results of the study by Keck et al. [2003b] are shown in Figure 30–3.) At endpoint, approximately half of the treated patients from both studies met response criteria for mania (≥50% reduction in Mania Rating Scale [MRS] scores).
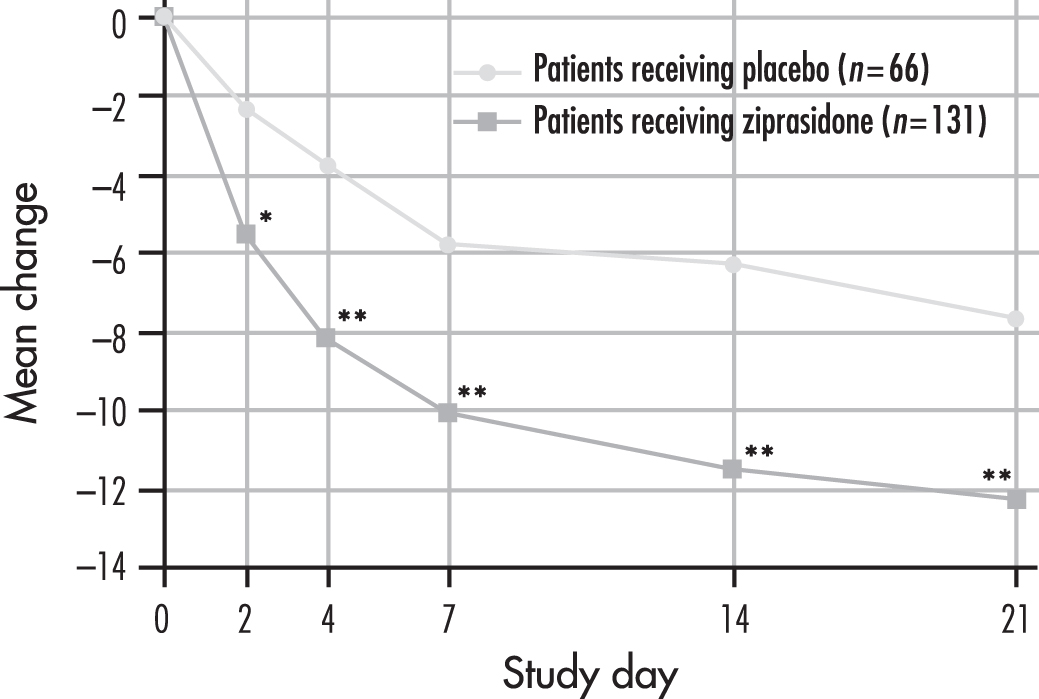
FIGURE 30–3. Effect of ziprasidone on mania: rating scale scores in patients with bipolar disorder receiving 21-day randomized treatment with ziprasidone or placebo.
*P<0.003 (F test), placebo-treated patients versus ziprasidone-treated patients.
**P<0.001 (F test), placebo-treated patients and ziprasidone-treated patients.
Source. Adapted from Keck et al. 2003b.
A number of placebo-controlled trials evaluating the efficacy of short-term monotherapy with various antipsychotics, including haloperidol, ziprasidone, olanzapine, risperidone, quetiapine, and aripiprazole, have demonstrated comparable improvement in symptoms of mania (Bowden et al. 2005; Hirschfeld et al. 2004; Keck et al. 2003a, 2003b; Khanna et al. 2005; McIntyre et al. 2005; McQuade et al. 2003; Potkin et al. 2005; Sachs et al. 2006; Smulevich et al. 2005; Tohen et al. 1999, 2000; Vieta et al. 2010; Weisler et al. 2003). Two large meta-analyses of randomized, placebo-controlled trials have examined the relative efficacy of various SGAs for the adjunctive treatment of mania (Perlis et al. 2006; Scherk et al. 2007). Although the statistical results of the two meta-analyses are similar, the authors of each study interpreted the results somewhat differently. Perlis et al. (2006) concluded that add-on therapy with SGAs (ziprasidone, olanzapine, quetiapine, and risperidone) conferred an additional benefit over monotherapy with a traditional mood stabilizer in reducing manic symptoms, with no difference in efficacy among the drugs. Scherk et al. (2007) also concluded that SGAs as a group were significantly superior to placebo as adjunctive treatment for mania but that ziprasidone and other individual agents may not be significantly superior to placebo in the adjunctive treatment of manic symptoms. This conclusion is in contrast with results reported by Vieta et al. (2010) from a 12-week double-blind two-part study in 438 adults with bipolar-associated acute mania. In the first part, a 3-week period in which ziprasidone (80–160 mg/day) and placebo were compared with haloperidol (8–30 mg/day) as a reference standard, changes from baseline MRS scores for ziprasidone and haloperidol were superior to those for placebo beginning with day 2 of treatment until week 3. At week 3, response rates were 36.9%, 54.7%, and 20.5%, respectively, for ziprasidone, haloperidol, and placebo. In the 9-week extension phase to examine tolerability, during which ziprasidone replaced placebo, improvements were maintained for 96.3% of patients receiving haloperidol and 88.1% of those receiving ziprasidone, with ziprasidone demonstrating superior tolerability.
Finally, regulatory approvals for the individual agents have supported the efficacy and safety of a number of individual antipsychotics for the acute treatment of mania, including ziprasidone (which is also approved for the acute treatment of mixed states). Prospective head-to-head comparator trials like that of Vieta et al. (2010) may further clarify whether differences in efficacy suggested by some meta-analyses are clinically informative or merely related to limitations in study design or methodology (e.g., underdosing of ziprasidone or dosing without food).
Bipolar Depression
Preliminary results indicate that ziprasidone may be a viable treatment option in bipolar depression. For example, an 8-week open-label study investigating ziprasidone monotherapy for depressive symptoms in bipolar II patients (n=30 completers) demonstrated effective attenuation of depression with a relatively low mean dosage of 58 mg/day (Liebowitz et al. 2009). The authors concluded that larger and controlled trials are required to confirm their findings.
Dysphoric Mania
Dysphoric mania is a common and often difficult-to-treat subset of bipolar mania that is associated with significant depressive symptoms. Data from a post hoc analysis of two similarly designed 3-week placebo-controlled trials (Stahl et al. 2010a) in acute bipolar mania that were pooled and analyzed indicated that ziprasidone significantly improved both depressive and manic mood symptoms in patients with dysphoric mania. A meta-analysis by Muralidharan et al. (2013) suggested that SGAs, including ziprasidone, are effective for the treatment of mixed states of bipolar disorder with predominant manic symptoms. A number of trials could not be included because data for mixed episodes were not presented separately. Similar to the preliminary results obtained in bipolar depression, definitive conclusions await further prospective controlled trials.
Maintenance Treatment
Two 52-week open-label extension studies support the safety, tolerability, and sustained efficacy of ziprasidone as maintenance treatment for bipolar disorder (P.E. Keck et al. 2004, 2009; Weisler et al. 2004). P.E. Keck et al. (2004) reported that treatment with ziprasidone (n=127; mean dosage, 123 mg/day) was associated with significantly lower MRS and CGI-S scores compared with baseline, beginning as early as the first week. Overall, improvements in manic symptoms achieved during acute treatment continued to consolidate during maintenance treatment with ziprasidone. During 52 weeks of treatment, only 6% of patients discontinued ziprasidone use because of relapse of mania. Similarly, only 4% of patients discontinued because of a clinical switch into depression. An important caveat regarding these results is the high rate of attrition observed by the end of 1 year, which is consistent with long-term studies involving other SGAs but still limits the full interpretation of results. Comparable results were observed in a separate extension study of adjunctive ziprasidone therapy (mean dosage, 92.6 mg/day) by Weisler et al. (2004); this study reported a mean improvement from baseline in MRS scores at all points throughout the study (Patel and Keck 2006). Finally, subjects with DSM-IV bipolar I disorder achieving 8 or more consecutive weeks of stability with open-label ziprasidone (80–160 mg/day) and lithium or valproate were randomly assigned in a 6-month double-blind maintenance period to receive ziprasidone plus mood stabilizer or placebo plus mood stabilizer. The time to intervention for a mood episode and the time to discontinuation for any reason were significantly longer for ziprasidone compared with placebo (Bowden et al. 2010), indicating that ziprasidone may be useful in combination with a classic mood stabilizer for maintenance of euthymia in bipolar disorder.
Treatment-Resistant Depression
A randomized, double-blind, placebo-controlled study, as well as a series of uncontrolled studies, has sparked interest in the efficacy of ziprasidone for depression, especially treatment-resistant depression (Barbee et al. 2004; Jarema 2007; Papakostas et al. 2004, 2015).
Papakostas et al. (2004) reported the results of a small study of 20 patients with major depression resistant to treatment with selective serotonin reuptake inhibitors (SSRIs). Open-label treatment with ziprasidone for 6 weeks, adjunctive to ongoing SSRI treatment, was evaluated with an intent-to-treat analysis that identified 10 treatment responders (defined as having a ≥50% decrease in depressive symptoms as measured by the 17-item version of the Hamilton Rating Scale for Depression (Ham-D-17). In order to further evaluate these effects, a randomized, double-blind, placebo-controlled trial was conducted to compare adjunctive ziprasidone with adjunctive placebo among 139 adult outpatients with major depressive disorder (MDD) that had not responded to an 8-week open-label trial of escitalopram alone (Papakostas et al. 2015). Compared with the patients randomly assigned to receive adjunctive placebo, those assigned to receive adjunctive ziprasidone (mean dosage, 98 mg/day; SD=40) with escitalopram demonstrated significantly greater rates of clinical response as well as significantly greater improvement on the Ham-D-17 Total score and the Hamilton Rating Scale for Anxiety score. Ziprasidone has also been tested in non-treatment-resistant depression. A 12-week randomized, double-blind, placebo-controlled sequential parallel comparison trial of ziprasidone as monotherapy for MDD in 120 outpatients with depression conducted by Papakostas et al. (2012) showed no significant efficacy for ziprasidone compared with placebo. The authors suggested that a larger trial may be required to detect significant differences.
Agitation
The efficacy of intramuscular ziprasidone for the treatment of agitated psychosis has been demonstrated in two randomized double-blind trials (2 mg intramuscular vs. 10 mg or 20 mg intramuscular, respectively, with up to three more doses allowed as needed at 4-hour or 2-hour intervals, respectively), leading to regulatory approval by the FDA (Daniel et al. 2001; Lesem et al. 2001). Treatment with single 10- or 20-mg doses leads to rapid reductions in symptom severity, with most patients having remission of agitation within 1 hour of dosing. Treatment with intramuscular ziprasidone is associated with a relatively low rate of concomitant benzodiazepine use (<20%) and may be better tolerated than haloperidol (Zhang et al. 2013). Sequential use of intramuscular ziprasidone followed by oral ziprasidone for the treatment of acute psychotic agitation has demonstrated superior efficacy, compared with sequential use of intramuscular and oral haloperidol, in two 7-day randomized open-label trials (Brook et al. 2000; Swift et al. 1998) as well as in a 6-week randomized, single-blind, flexible-dose study (Brook et al. 2005). Clinical improvement occurred more rapidly than with haloperidol in one study and as quickly as 30 minutes after the first intramuscular administration of ziprasidone (Swift et al. 1998). Cumulative data from these studies indicate that intramuscular ziprasidone can rapidly control agitation and psychotic symptoms and provide greater mean improvements in acute agitation than seen with intramuscular haloperidol (e.g., greater mean improvements in BPRS total score, agitation, and CGI-S score) (Brook 2003).
Pediatric Use
Ziprasidone is not approved in the United States by the FDA for use in pediatric patients. Its use has been evaluated in children and adolescents (ages 10–17 years) experiencing manic or mixed episodes associated with bipolar disorder (Kuehn 2009). In the European Union it has been approved for the treatment of bipolar disorder (mania) in pediatric patients. Results from RCTs of ziprasidone in children and adolescents (ages 10–17 years) with bipolar disorder (Versavel et al. 2005) and with bipolar disorder, schizophrenia, or schizoaffective disorder (DelBello et al. 2008) have been reported. In the former study, treatment with ziprasidone was associated with improvement in mania and overall psychopathology (Versavel et al. 2005). The latter study focused on safety and did not report any unexpected tolerability findings in this age population, using a starting dosage of 20 mg/day titrated to between 80 and 160 mg/day over 1–2 weeks for clinically determined optimal dosing (DelBello et al. 2008). Finally, a 4-week acute randomized, placebo-controlled multicenter trial, followed by a 26-week open-label extension study, in pediatric patients with bipolar disorder (ages 10–17 years) showed benefits of ziprasidone and a benign short-term side-effect profile (Findling et al. 2013a). In pediatric schizophrenia, in a study using a 6-week randomized (2:1), double-blind, placebo-controlled design, ziprasidone did not separate from placebo (Findling et al. 2013b).
Side Effects and Toxicology
Tolerability Profile in Clinical Trials
Ziprasidone has a favorable tolerability profile based on both short- and long-term clinical trials (Daniel 2003; Pfizer Inc. 2004). The four most common treatment-related adverse events associated with oral ziprasidone in short-term premarketing placebo-controlled trials for schizophrenia were somnolence (14%), EPS (14%), nausea (10%), and constipation (9%) (Pfizer Inc. 2008). In subsequent clinical trials, treatment with ziprasidone was associated with a low occurrence of adverse events, most of which were considered mild to moderate in severity (Arango et al. 2007; Arato et al. 2002; Lieberman 2007; Nemeroff et al. 2005; Weiden et al. 2002, 2003b). In addition to the comprehensive listing of potential adverse events available in the full U.S. prescribing information (USPI) (Pfizer Inc. 2008), published case reports offer accounts of various rare adverse events that may be associated with the use of ziprasidone (Akkaya et al. 2006; Kaufman et al. 2006; Miodownik et al. 2005; Murty et al. 2002; Villanueva et al. 2006).
Intramuscular ziprasidone shows a favorable tolerability profile similar to that of oral ziprasidone. In premarketing trials of intramuscular ziprasidone, the most common side effects (those with an incidence of >5% and an incidence greater than that seen in placebo recipients) were somnolence (20%), headache (13%), and nausea (12%) (Pfizer Inc. 2008). Pooled data from clinical trials of intramuscular ziprasidone indicate that most treatment-related adverse events were mild to moderate in severity, with the most common side effects being headache, nausea, dizziness, insomnia, anxiety, and pain at the injection site (Daniel 2003; Zimbroff et al. 2002).
Safety in Pregnancy
Ziprasidone is considered a Category C drug in pregnancy. Although some specific developmental effects have been noted in animal studies at dosages ranging from 0.5 to 8.0 times the maximal recommended human dosage (Pfizer Inc. 2008), there are as yet no similar reports of such effects in humans. The reader is advised to consult the current USPI for a detailed listing of potential adverse drug effects identified in the regulatory approval process and postmarketing surveillance.
Increased Mortality Risk in Elderly Patients With Dementia-Related Psychosis
In 2005, the FDA mandated the addition of a black box warning in the USPI regarding an increased risk of mortality associated with the use of both SGAs and FGAs in elderly patients with dementia-related psychosis. Observed causes of death have been varied, and the mechanism of any drug effect in schizophrenia remains uncertain (Pfizer Inc. 2008). In particular, it remains unclear to what extent these uncontrolled observations of increased mortality are due to specific drug effects or to the advanced medical risk characteristics of patients with dementia or delirium who tend to receive these medications (Farber et al. 2000; Rochon et al. 2008). A recent retrospective cohort study among antipsychotic-exposed older adults did not find increased mortality associated with ziprasidone, in contrast with haloperidol, olanzapine, and risperidone (Gerhard et al. 2014).
Retrospective analysis of intramuscular use of ziprasidone, olanzapine, or haloperidol in large matched cohorts of hospitalized patients did not indicate significant differences in mortality rates among the three cohorts (Holdridge et al. 2010).
Activation Effects
Clinical experience with ziprasidone in the years following initial U.S. approval has suggested that a small subgroup of patients may experience insomnia, or what has been characterized as activation or akathisia, soon after initiation of treatment (Nemeroff et al. 2005). These presentations have been described as transient manifestations of anxiety, restlessness, insomnia, increased energy, or hypomania-like symptoms, occurring most commonly at what is now considered the lower end of the dosage range. Anecdotal reports suggest that starting dosages of 120 mg/day or greater and more rapid dose titration can substantially reduce the incidence of these clinical presentations (Weiden et al. 2002). These anecdotal clinical observations are consistent with controlled experimental evidence indicating that a significantly lower rate of discontinuation occurs in patients who begin ziprasidone therapy at higher dosages (120–160 mg/day) than in patients who receive initial dosages of 80 mg/day or less (Joyce et al. 2006). Several mechanisms may explain these observations. First, ziprasidone is less intrinsically sedating than many other antipsychotics in current use (e.g., due to less H1 receptor antagonism), so that patients initiating ziprasidone treatment after months or years of receiving a more sedating therapy may experience initial difficulties adjusting to the new level of drug-related sedation. Second, as discussed earlier in this chapter (see “Pharmacological Profile” and “Indications and Efficacy” sections), many patients have been treated with ziprasidone at dosages that were insufficient to achieve optimal D2 receptor blocking, leading to undertreatment of the underlying illness compared with what might have been achieved with an appropriately dosed prior therapy.
Furthermore, ziprasidone underdosing with respect to D2 receptor binding can produce a well-understood but unwanted pharmacodynamic situation with respect to the differential balance of 5-HT2C receptor antagonism relative to D2 receptor antagonism. As was illustrated in Figure 30–2, use of ziprasidone dosages at the lower end of the clinical dosage range can allow 5-HT2 receptors to reach 50% or greater maximal receptor occupancy well before clinically significant levels of D2 receptor occupancy are achieved (Mamo et al. 2004). 5-HT2C receptor antagonist activity at this level disinhibits cortical monoaminergic neurotransmission (e.g., dopamine release), which, in the absence of sufficient D2 receptor blockade, may lead to clinically relevant excess monoaminergic neurotransmission (Bonaccorso et al. 2002; Pozzi et al. 2002). Clinicians commonly address these potential issues through appropriate dosing and through the transient targeted use of concomitant medication strategies (e.g., adjunctive benzodiazepine treatment) for relevant patients starting new treatment in the acute inpatient setting or for stable outpatients needing a smooth transition to new therapy.
DRESS Syndrome
In 2014, the FDA issued a warning that ziprasidone is one of more than 50 medications associated with a rare condition called Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome. This potentially life-threatening hypersensitivity reaction presents as a skin rash with fever, eosinophilia, lymphadenopathy, and multiorgan inflammation. Patients who develop this constellation of symptoms during treatment with ziprasidone should be evaluated for possible DRESS syndrome, and if that syndrome is suspected, their medication should be discontinued (Cacoub et al. 2011).
Extrapyramidal, Metabolic, and Cardiac Adverse Effects
Extrapyramidal Side Effects
Short-term trials indicate that treatment with ziprasidone is associated with a larger incidence of EPS compared with treatment with placebo (Pfizer Inc. 2008; Potkin et al. 2005). In contrast, data from a 52-week trial (Arato et al. 2002) indicate that the incidence of abnormal movement disorders during treatment with ziprasidone is comparable to the incidence during placebo treatment. Other long-term studies suggest a low (<6%) incidence of treatment-related EPS (Arango et al. 2007; Kudla et al. 2007). Both active-comparator studies and medication-switching studies suggest that ziprasidone is associated with fewer EPS than risperidone (Addington et al. 2003, 2009; Weiden et al. 2003a, 2003b) or FGAs (Hirsch et al. 2002; Weiden et al. 2003a, 2003b). A direct comparison study of ziprasidone and olanzapine, a drug with intrinsic antimuscarinic activity as well as 5-HT2 receptor antagonist activity, reported that treatment with olanzapine was associated with fewer EPS (Kinon et al. 2006), whereas two other direct comparison studies (Breier et al. 2005; Simpson et al. 2002) and one medication-switching study (Weiden et al. 2003b) reported that the two drugs exhibited similar liability for EPS. With respect to akathisia, one comparison study indicated that less akathisia occurs with olanzapine use (Breier et al. 2005), whereas another study noted no difference in akathisia rates with olanzapine versus ziprasidone (Kinon et al. 2006). Results from phase 1 and phase 2 of the large-scale CATIE study suggested no significant differences among ziprasidone, perphenazine, olanzapine, quetiapine, and risperidone in the incidence of EPS and akathisia (Lieberman 2007; Lieberman et al. 2005; Stroup et al. 2006). However, the perphenazine arm in the CATIE study was restricted to patients who did not already have tardive dyskinesia, suggesting a possible selection bias toward patients less likely to experience EPS. Despite this advantage, the perphenazine group still had the highest rate of dropouts due to EPS. Investigating SGAs only, a recent meta-analysis (Rummel-Kluge et al. 2012) of 54 comparative clinical trials analyzed the use of antiparkinsonian medication as an indicator of treatment-related EPS. The authors reported that risperidone was associated with greater use of antiparkinsonian medication compared with clozapine, olanzapine, quetiapine, or ziprasidone. Ziprasidone was associated with more use of antiparkinsonian medication than olanzapine or quetiapine; however, the authors concluded that overall differences were small. EUFEST data (Rybakowski et al. 2014) suggest that in first-episode schizophrenia patients during the first year of antipsychotic treatment (including quetiapine), EPS were present at low and manageable levels. More recently, SGAs in patients never exposed to FGAs have been examined for their tardive movement disorder liability (Ryu et al. 2015). Although the incidence of movement disorder symptoms was low, the authors found that the median interval between the first exposure to the antipsychotic and movement syndrome onset was 15 months for tardive dyskinesia and 43 months for tardive dystonia.
Intramuscular ziprasidone has been tolerated at dosages of up to 80 mg/day with a low liability for EPS (Daniel 2003; Daniel et al. 2001; Lesem et al. 2001). Both intramuscular ziprasidone use and sequential intramuscular/oral ziprasidone use are associated with a lower incidence of treatment-related movement disorders than intramuscular haloperidol use (Swift et al. 1998; Zimbroff et al. 2002) and sequential intramuscular/oral haloperidol use (Brook et al. 2000, 2005). Although the results of controlled experimental studies indicate a generally low risk of EPS with ziprasidone, there have been uncontrolled observational reports of EPS-related adverse events co-occurring with ziprasidone treatment and, in many cases, concomitant treatment with other agents (Dew and Hughes 2004; Duggal 2007; Keck et al. 2004; Mason et al. 2005; Papapetropoulos et al. 2005; Ramos et al. 2003; Rosenfield et al. 2007; Weinstein et al. 2006; Yumru et al. 2006; Ziegenbein et al. 2003).
Metabolic Adverse Effects
Adverse medication effects on modifiable risk factors for cardiovascular disease and type 2 diabetes mellitus have become an important topic of clinical, research, and regulatory concern, based in part on the increased prevalence of these conditions and associated premature mortality in patients with major mental disorders (Brown 1997; Brown et al. 2000; Colton and Manderscheid 2006; Harris and Barraclough 1998; Hennekens et al. 2005; Joukamaa et al. 2001; Osby et al. 2000, 2001). Modifiable cardiometabolic risk factors include obesity, hyperglycemia, dyslipidemia, hypertension, and smoking—all prevalent conditions in patients with major mental disorders, with substantial evidence that primary and secondary prevention approaches are underutilized in these patients (Allison et al. 1999a; Brown et al. 2000; Druss and Rosenheck 1998; Druss et al. 2000, 2001; Frayne et al. 2005; Hippisley-Cox et al. 2007; McEvoy et al. 2005; Nasrallah et al. 2006; Newcomer and Hennekens 2007). In particular, use of recommended monitoring of changes in weight and in plasma glucose and lipid levels during antipsychotic treatment has heightened interest in cardiometabolic risk effects that may go undetected during the course of treatment (American Diabetes Association 2004; Morrato et al. 2008). All currently available antipsychotic medications are associated with a risk of weight gain, as well as potential adverse effects on plasma glucose and lipid levels, although there is substantial variability in the magnitude of these effects across individual agents (Casey et al. 2004; Eli Lilly 2008; Newcomer 2005; Schizophrenia.com 2007). Potential adverse treatment effects on body weight can increase the risk for cardiovascular disease and type 2 diabetes, commonly via adiposity-related increases in insulin resistance, dyslipidemia, and hyperglycemia (Fontaine et al. 2001; Haupt et al. 2007; Koro et al. 2002a, 2002b).
Treatment with ziprasidone is associated with a relatively low risk of clinically significant increases in body weight. An analysis of available studies with this agent and other antipsychotics, both FGAs and SGAs (Allison et al. 1999b), estimated a mean 0.04-kg weight gain over a 10-week treatment period with ziprasidone, identifying ziprasidone as having one of the lowest estimated effects on body weight of those analyzed. In a 6-week RCT in patients with acute exacerbations of schizophrenia or schizoaffective disorder, treatment with ziprasidone 80 mg/day produced a median increase in body weight of 1 kg, compared with no change in median weight with ziprasidone 160 mg/day or placebo (Daniel et al. 1999). In a 28-week study of outpatients with schizophrenia, mean changes in body weight from baseline to endpoint were similar during treatment with ziprasidone (+0.31 kg) and haloperidol (+0.22 kg) (Hirsch et al. 2002). In a 28-week study comparing the effects of ziprasidone and olanzapine, ziprasidone-treated patients experienced a small decrease in mean body weight (−1.12 kg) compared with a statistically and clinically different 3.06-kg mean increase in body weight observed with olanzapine treatment (Hardy et al. 2003; Kinon et al. 2006). Reductions in body weight were also associated with ziprasidone treatment in the 1-year ZEUS study of patients with chronic, stable schizophrenia (Arato et al. 2002); this study reported mean decreases from baseline of 2.7 kg, 3.2 kg, and 2.9 kg with 40 mg/day, 80 mg/day, and 160 mg/day dosages of ziprasidone, respectively, compared with a 3.6-kg decrease observed with placebo treatment. Results from the phase 1 and phase 2 CATIE studies provide further confirmation that treatment with ziprasidone has a low intrinsic risk for producing clinically significant weight gain, with 6%–7% of ziprasidone recipients demonstrating a 7% or greater increase from baseline body weight compared with, for example, 27%–30% of olanzapine recipients (Lieberman 2007; Stroup et al. 2006). In the phase 1 CATIE study, ziprasidone treatment was associated with a mean reduction in body weight of 0.14 kg (0.3 lb) per month of treatment, compared with a mean increase of 0.91, 0.23, and 0.18 kg per month (2.0, 0.5, and 0.4 lb per month, respectively) during treatment with olanzapine, quetiapine, and risperidone, respectively, the other SGAs tested (Lieberman et al. 2005). A recent meta-analysis of pooled data from nine placebo-controlled RCTs suggested no significant differences in weight gain between patients treated with ziprasidone versus those receiving placebo (Gao et al. 2013).
It is important to note that initial courses of treatment can clearly be associated with greater weight gain than subsequent courses of treatment (McEvoy et al. 2007). In addition, chronically treated patients switching treatment from a medication with greater weight gain liability to a medication with less weight gain liability are likely to lose body weight in relation to that medication change, an effect that likely underlies the mean reductions in weight noted in some of the trials with ziprasidone discussed above. The magnitude of change in body weight during treatment with ziprasidone varies as a function of the weight gain liability of the prior treatment: the greatest potential for weight loss is associated with switching from previous treatments with the greatest weight gain liability (Weiden et al. 2008). For example, 6 weeks of ziprasidone therapy was associated with statistically significant decreases in mean body weight from baseline in patients switched from olanzapine (−1.8 kg) and from risperidone (−0.9 kg), whereas patients switched from high-potency FGAs such as haloperidol experienced a small increase in weight (+0.3 kg) (Weiden et al. 2008). The 1-year extension of this medication-switching study indicated that weight loss was progressive and persistent throughout the 1-year period for patients who switched from olanzapine (−9.8 kg, or 10.3% of baseline body weight) and from risperidone (−6.9 kg, or 7.8% of baseline) (Figure 30–4; Weiden et al. 2008). Another study found significant decreases in weight in patients treated for 6 months with ziprasidone who were switched from olanzapine (−7.0 kg) and from risperidone (−2.2 kg) (Montes et al. 2007).
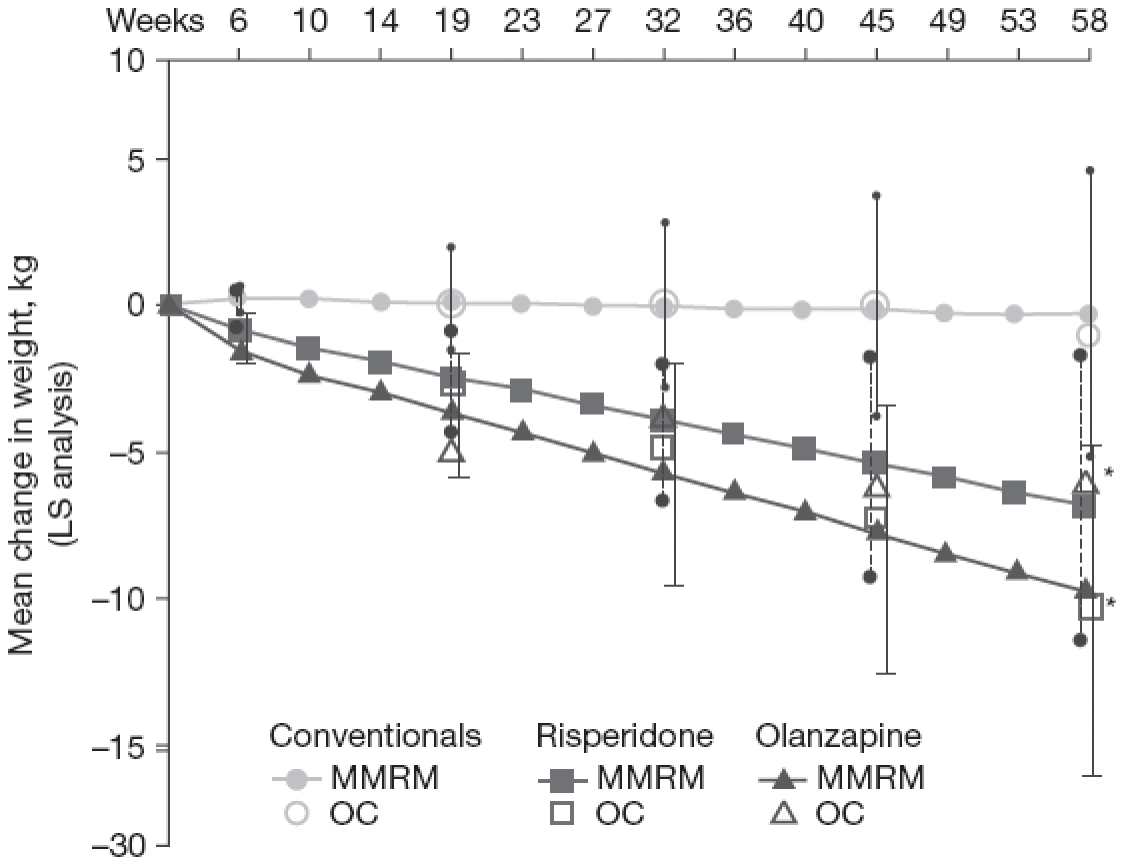
FIGURE 30–4. Time course of weight change over 58 weeks after switching to ziprasidone.
Previous treatments were conventional antipsychotics (line with circles; n=71), risperidone (line with squares; n=43), or olanzapine (line with triangles; n=71). Individual observed cases within each treatment group are also shown (circle=conventional agent: baseline weight, 198 lbs [90 kg]; square=risperidone: baseline weight, 194.9 lbs [88.6 kg]; triangle=olanzapine: baseline weight, 210.3 lbs [95.6 kg]). LS=least-squares analysis; MMRM=mixed-model repeated-measures analysis; OC=observed case analysis.
*P<0.01 versus baseline (MMRM and OC).
Source. Adapted from Weiden et al. 2008, Figure 1 (p. 988).
Ziprasidone’s effects on plasma glucose and lipid levels are best understood as being a function of treatment-related changes in adiposity. Whereas some antipsychotics, such as clozapine and olanzapine, have been reported to produce adiposity-independent effects on insulin sensitivity and related changes in glucose and lipid metabolism, ziprasidone has demonstrated no similar adiposity-independent effects in this same experimental paradigm (Houseknecht et al. 2007). In general, increases in adiposity are associated with decreases in insulin sensitivity in individuals taking or not taking antipsychotic medications, with reduced insulin sensitivity leading to increased risk for hyperglycemia, dyslipidemia, and other adverse changes in cardiometabolic risk indicators (Haupt et al. 2007; Newcomer and Haupt 2006; Strassnig et al. 2015).
Both short- and long-term studies have shown minimal adverse effects of ziprasidone on glucose levels, plasma insulin levels, insulin resistance, or fasting and nonfasting lipid levels (Daniel et al. 1999; Glick et al. 2001; Rettenbacher et al. 2006; Simpson et al. 2004a, 2005), in contrast to the degree of adverse effects detected with some active comparators. For example, olanzapine treatment can produce statistically significant increases in fasting glucose and insulin levels (Glick et al. 2001; Hardy et al. 2003; Simpson et al. 2002, 2005). In the CATIE phase 1 study, ziprasidone treatment was associated with minimal drug exposure–adjusted mean increases in blood glucose (+2.9±3.4 mg/dL) and hemoglobin A1c (HbA1c) (+0.11 ±0.09%) and decreases in plasma triglycerides (−16.5±12.2 mg/dL) and total cholesterol (−8.2±3.2 mg/dL) (Lieberman 2007). In the CATIE phase 2 study, ziprasidone-treated patients showed minimal drug exposure–adjusted mean increases in blood glucose (+0.8±5.6 mg/dL) and HbA1c (+0.46±0.3%) and decreases in triglycerides (−3.5±20.9 mg/dL) and total cholesterol (−10.7±5.1 mg/dL) (Stroup et al. 2006).
Similar to the effect of prior treatment conditions on changes in weight during treatment with ziprasidone, improvements in plasma lipid levels observed during ziprasidone treatment in the CATIE study can best be understood as the effect of switching from a previous treatment that is associated with larger adverse effects on lipid metabolism to a treatment with minimal adverse effects. Weiden et al. (2003a) noted that ziprasidone treatment was associated with significant decreases from baseline in both median nonfasting triglyceride levels and median nonfasting total cholesterol levels at the end of the 6-week treatment period in patients whose prior medication was olanzapine or risperidone, with minimal change following prior treatment with high-potency FGAs like haloperidol. Notably, the reductions in lipids observed during this study occurred within the first 6 weeks of initiating treatment with ziprasidone, with substantial reductions in total cholesterol (>20 mg/dL) and plasma triglycerides (78 mg/dL) in the patients previously treated with olanzapine. In the 12-month extension of this study, the reductions achieved in the initial weeks following the switch from prior treatment were sustained during continued treatment with ziprasidone (Weiden et al. 2008).
Cardiac Effects and Risks
Some medications, including psychotropic medications, can increase the duration of the QTc interval (i.e., the QT interval corrected for heart rate). Basic research suggests plausible mechanisms by which an increase in the QTc interval could increase the risk of sudden cardiac death, and clinical investigations suggest that certain small subgroups of the general population may have an increased risk of sudden cardiac death—for example, those with a family history of congenital long-QT syndrome (>500 msec) and those who concomitantly use drugs that markedly increase the QTc interval (e.g., by >60 msec) via either pharmacokinetic or pharmacodynamic interactions (Montanez et al. 2004). These potential risks have understandably led to regulatory interest in drug effects on the QTc interval. It should be noted that epidemiological studies in the general population suggest that modest prolongations of the QTc interval are not a risk factor for cardiovascular mortality or sudden death, so any risk in the general population of modest QTc prolongations is likely to be small and difficult to detect reliably (Montanez et al. 2004). When considered alongside risks such as obesity, hypercholesterolemia, diabetes, hypertension, physical inactivity, or cigarette smoking, each with well-characterized effects in the general population, modest QTc prolongations do not represent a comparable risk factor for cardiovascular mortality or sudden death in the general population.
With this background, thioridazine, following decades of use, was required to add to its prescribing information a black box warning related to its QTc interval–prolonging effects. Other FGAs, including haloperidol, are also associated with some risk of QTc interval prolongation (Gury et al. 2000; O’Brien et al. 1999). The rate of occurrence of torsades de pointes with FGAs has been estimated as “10–15 such events in 10,000 person-years of observation” (Glassman and Bigger 2001, p. 1774). Ziprasidone, in common with certain other antipsychotic agents, can induce orthostatic hypotension, particularly early in treatment exposure, which can lead to transient tachycardia, dizziness, or syncope (Swainston Harrison and Scott 2006). However, tachycardia has been observed to be infrequent and to be as common in patients treated with placebo as in those treated with ziprasidone (Swainston Harrison and Scott 2006). Tachycardia and syncope related to hypotension are to be distinguished from ventricular arrhythmias that in rare cases can occur in relation to QTc interval prolongation.
Ziprasidone treatment has been demonstrated to result in a modestly increased risk of QTc interval prolongation (Pfizer Inc. 2008). This QTc interval prolongation at Cmax (mean increase, >15 msec) is 9–14 msec greater than that seen with risperidone, olanzapine, quetiapine, or haloperidol but approximately 14 msec less than that seen with thioridazine. Unlike the case with thioridazine, the modest effect of ziprasidone on the QTc interval is not worsened by the presence of commonly encountered inhibitors of drug metabolism. In clinical trials of ziprasidone monotherapy that reported QTc interval changes, in studies of high-dosage intramuscular administration (Miceli et al. 2010), and in case reports of ziprasidone overdose, there was no evidence of any significant clinical sequelae such as torsades de pointes or sudden death (Arato et al. 2002; Arbuck 2005; Daniel 2003; Gómez-Criado et al. 2005; Harrigan et al. 2004; Insa Gómez and Gutiérrez Casares 2005; Levy et al. 2004; Lieberman 2007; Miceli et al. 2004; Montanez et al. 2004; Nemeroff et al. 2005; Tan et al. 2009; Taylor 2003; Weiden et al. 2002, 2003a). These findings are consistent with analyses of large population samples, which have failed to demonstrate any association between QTc interval duration and either cardiovascular or all-cause mortality (Goldberg et al. 1991).
The Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC) was a large (>18,000 participants) international randomized trial designed to examine the risks of nonsuicide mortality and hospitalization associated with ziprasidone’s use in routine medical practice settings (Strom et al. 2011). The main objective of ZODIAC was to evaluate nonsuicide mortality in the year following treatment initiation, which limited the study’s ability to provide data on drug efficacy. Nevertheless, findings did not show an elevated risk of nonsuicide mortality for ziprasidone relative to olanzapine, and the study excluded a relative risk larger than 1.39 with a high probability. A total of 205 deaths occurred in the overall study population (N=18,154). It should be noted that ZODIAC was not designed to measure electrocardiographic parameters or to examine the risk of rare cardiac events associated with lengthening of the QTc interval. A recent meta-analysis evaluating the cardiac safety of antipsychotics in pediatric patients (Jensen et al. 2015) found a mean increase in QTc interval of 8.74 ms with ziprasidone. Compared with placebo, none of the tested antipsychotics caused a significant increase in the incidence of QTc prolongation, although there was significant reporting bias. The authors concluded that individual risk factors need to be taken into account when evaluating medication-related QTc interval changes.
Rare cases of torsades de pointes have been reported with a number of different medications, including ziprasidone, but the incidence of these events appears to be below the known prevalence of torsades de pointes in community-based population samples (Heinrich et al. 2006). The USPI suggests that clinicians should nonetheless be cognizant of this potential risk and be aware of circumstances that may increase the risk of torsades de pointes and/or sudden death in association with the use of any drugs that can prolong the QTc interval. Such circumstances include bradycardia, hypokalemia, or hypomagnesemia; concomitant use of other medications known to cause clinically significant QT prolongation (although an additive effect with ziprasidone has not been established); and presence of congenital long-QT syndrome. The USPI further states that ziprasidone should not be used in patients who have significant cardiovascular conditions, such as uncompensated heart failure or a cardiac arrhythmia, or in those who have had a recent acute myocardial infarction or persistent QTc interval measurements of greater than 500 msec, and the prudent clinician might consider employing the same caution with many other antipsychotic and psychotropic medications currently in use. A comprehensive review of the cardiac safety of antipsychotics (Hasnain and Vieweg 2014) reached a similar conclusion—that little evidence exists that antipsychotic drug–associated QTc interval prolongation is in itself sufficient to cause torsades de pointes in the absence of other factors that facilitate or attenuate progression of drug-associated QTc interval prolongation to torsades de pointes.
Drug–Drug Interactions
A study of in vitro enzyme inhibition (Pfizer Inc. 2008) indicated that ziprasidone has very little inhibitory effect on CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 and is thus unlikely to interfere with the metabolism of other medications relying on these enzymes for clearance. In vivo studies indicated that ziprasidone has no effect on the pharmacokinetics of lithium, estrogen, progesterone, or dextromethorphan (Pfizer Inc. 2008). As noted earlier (in the “Metabolism and Elimination” subsection), less than one-third of ziprasidone clearance is mediated by CYP-catalyzed oxidation, and because of this, one would not anticipate a substantial change in ziprasidone AUC during coadministration with CYP3A4 inhibitors or inducers. Aldehyde oxidase–mediated reduction constitutes the primary metabolic pathway for ziprasidone. As also noted above, currently no clinically significant pharmacological inhibitors of aldehyde oxidase are commonly encountered (Obach et al. 2004).
Consistent with this prediction, coadministration of ziprasidone with ketoconazole, a potent CYP3A4 inhibitor, results in only a modest increase in ziprasidone AUC (33%) and Cmax (34%) (Miceli et al. 2000b), whereas coadministration with carbamazepine, an inducer of 3A4, results in modest reductions in ziprasidone AUC (44%) and Cmax (39%) (Miceli et al. 2000a). Coadministration with CYP2D6 inhibitors has no effect on ziprasidone plasma levels. Coadministration of ziprasidone with lithium results in no significant change in steady-state lithium levels (Apseloff et al. 2000), and commonly used antacids and cimetidine do not significantly alter ziprasidone pharmacokinetics (Pfizer Inc. 2008).
Conclusion
Ziprasidone was the fourth atypical antipsychotic following clozapine to become available in the United States. This agent has a unique pharmacological profile, with the highest 5-HT2A/D2 affinity ratio among currently available agents, potent serotonin and norepinephrine reuptake inhibition activity, agonist activity at 5-HT1A receptors, and clinically relevant antagonist activity at various 5-HT2 receptor subtypes. Ziprasidone has demonstrated rapid-onset and sustained efficacy for the treatment of schizophrenia, schizoaffective disorder, and bipolar mania, with promising evidence of favorable mood, cognitive, and prosocial effects. It is also available in an intramuscular formulation for the treatment of acute agitated psychoses, and it was approved for the use of bipolar mania in children and adolescents 10–17 years of age.
Ziprasidone has highly favorable safety and tolerability profiles with limited potential for drug–drug and drug–disease interactions—critical issues for a patient population that generally has a high burden of medical comorbidity and is commonly exposed to complex polypharmacy. The adverse-effect profile of ziprasidone is particularly noteworthy in areas that are key to safety and tolerability in patients with major mental disorders such as schizophrenia and bipolar disorder, including low drug-related risk for acute EPS and minimal effects on cardiometabolic risk factors such as obesity and dyslipidemia.
References
Addington D, Pantelis C, Dineen M, et al: Ziprasidone vs risperidone in schizophrenia: 52 weeks’ comparison. Poster presented at the annual meeting of the American Psychiatric Association, San Francisco, CA, May 17–22, 2003
Addington DE, Pantelis C, Dineen M, et al: Efficacy and tolerability of ziprasidone versus risperidone in patients with acute exacerbation of schizophrenia or schizoaffective disorder: an 8-week, double-blind, multicenter trial. J Clin Psychiatry 65(12):1624–1633, 2004 15641867
Addington DE, Labelle A, Kulkarni J, et al: A comparison of ziprasidone and risperidone in the long-term treatment of schizophrenia: a 44-week, double-blind, continuation study. Can J Psychiatry 54(1):46–54, 2009 19175979
Akkaya C, Sarandol A, Sivrioglu EY, et al: A patient using ziprasidone with polydipsia, seizure, hyponatremia and rhabdomyolysis. Prog Neuropsychopharmacol Biol Psychiatry 30(8):1535–1538, 2006 16820256
Allison DB, Fontaine KR, Heo M, et al: The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry 60(4):215–220, 1999a 10221280
Allison DB, Mentore JL, Heo M, et al: Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 156(11):1686–1696, 1999b 10553730
Altar CA, Wasley AM, Neale RF, et al: Typical and atypical antipsychotic occupancy of D2 and S2 receptors: an autoradiographic analysis in rat brain. Brain Res Bull 16(4):517–525, 1986 2872945
American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity: Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 27(2):596–601, 2004 14747245
Andreasen NC, Carpenter WT Jr, Kane JM, et al: Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 162(3):441–449, 2005 15741458
Apseloff G, Mullet D, Wilner KD, et al: The effects of ziprasidone on steady-state lithium levels and renal clearance of lithium. Br J Clin Pharmacol 49 (suppl 1): 61S–64S, 2000 10771456
Arango C, Kirkpatrick B, Koenig J: At issue: stress, hippocampal neuronal turnover, and neuropsychiatric disorders. Schizophr Bull 27(3):477–480, 2001 11596848
Arango C, Gómez-Beneyto M, Brenlla J, et al; ZIS Study Group: A 6-month prospective, observational, naturalistic, uncontrolled study to evaluate the effectiveness and tolerability of oral ziprasidone in patients with schizophrenia. Eur Neuropsychopharmacol 17(6–7):456–463, 2007 17234389
Arato M, O’Connor R, Meltzer HY; ZEUS Study Group: A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the Ziprasidone Extended Use in Schizophrenia (ZEUS) study. Int Clin Psychopharmacol 17(5):207–215, 2002 12177583
Arbuck DM: 12,800-mg ziprasidone overdose without significant ECG changes. Gen Hosp Psychiatry 27(3):222–223, 2005 15882771
Bagnall AM, Jones L, Ginnelly L, et al: A systematic review of atypical antipsychotic drugs in schizophrenia. Health Technol Assess 7(13):1–193, 2003 12925268
Barbee JG, Conrad EJ, Jamhour NJ: The effectiveness of olanzapine, risperidone, quetiapine, and ziprasidone as augmentation agents in treatment-resistant major depressive disorder. J Clin Psychiatry 65(7):975–981, 2004 15291687
Beedham C, Miceli JJ, Obach RS: Ziprasidone metabolism, aldehyde oxidase, and clinical implications. J Clin Psychopharmacol 23(3):229–232, 2003 12826984
Bonaccorso S, Meltzer HY, Li Z, et al: SR46349-B, a 5-HT(2A/2C) receptor antagonist, potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Neuropsychopharmacology 27(3): 430–441, 2002 12225700
Bowden CL, Grunze H, Mullen J, et al: A randomized, double-blind, placebo-controlled efficacy and safety study of quetiapine or lithium as monotherapy for mania in bipolar disorder. J Clin Psychiatry 66(1):111–121, 2005 15669897
Bowden CL, Vieta E, Ice KS, et al: Ziprasidone plus a mood stabilizer in subjects with bipolar I disorder: a 6-month, randomized, placebo-controlled, double-blind trial. J Clin Psychiatry 71(2):130–137, 2010 20122373
Breier A, Berg PH, Thakore JH, et al: Olanzapine versus ziprasidone: results of a 28-week double-blind study in patients with schizophrenia. Am J Psychiatry 162(10): 1879–1887, 2005 16199834
Bremner JD, Vythilingam M, Ng CK, et al: Regional brain metabolic correlates of alpha-methylparatyrosine-induced depressive symptoms: implications for the neural circuitry of depression. JAMA 289(23):3125–3134, 2003 12813118
Briley M, Moret C: Neurobiological mechanisms involved in antidepressant therapies. Clin Neuropharmacol 16(5):387–400, 1993 8221701
Brook S: Intramuscular ziprasidone: moving beyond the conventional in the treatment of acute agitation in schizophrenia. J Clin Psychiatry 64 (suppl 19):13–18, 2003 14728085
Brook S, Lucey JV, Gunn KP; Ziprasidone I.M. Study Group: Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. J Clin Psychiatry 61(12):933–941, 2000 11206599
Brook S, Walden J, Benattia I, et al: Ziprasidone and haloperidol in the treatment of acute exacerbation of schizophrenia and schizoaffective disorder: comparison of intramuscular and oral formulations in a 6-week, randomized, blinded-assessment study. Psychopharmacology (Berl) 178(4):514–523, 2005 15650846
Brown S: Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry 171:502–508, 1997 9519087
Brown S, Inskip H, Barraclough B: Causes of the excess mortality of schizophrenia. Br J Psychiatry 177:212–217, 2000 11040880
Bymaster FP, Katner JS, Nelson DL, et al: Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27(5):699–711, 2002 12431845
Cacoub P, Musette P, Descamps V, et al: The DRESS syndrome: a literature review. Am J Med 124(7):588–597, 2011 21592453
Casey DE, Haupt DW, Newcomer JW, et al: Antipsychotic-induced weight gain and metabolic abnormalities: implications for increased mortality in patients with schizophrenia. J Clin Psychiatry 65 (suppl 7):4–18, quiz 19–20, 2004 15151456
Citrome L: Using oral ziprasidone effectively: the food effect and dose-response. Adv Ther 26(8):739–748, 2009 19669631
Citrome L, Jaffe A, Levine J: How dosing of ziprasidone in a state hospital system differs from product labeling. J Clin Psychiatry 70(7):975–982, 2009a 19653974
Citrome L, Reist C, Palmer L, et al: Impact of real-world ziprasidone dosing on treatment discontinuation rates in patients with schizophrenia or bipolar disorder. Schizophr Res 115(2–3):115–120, 2009b 19864113
Citrome L, Yang R, Glue P, et al: Effect of ziprasidone dose on all-cause discontinuation rates in acute schizophrenia and schizoaffective disorder: a post-hoc analysis of 4 fixed-dose randomized clinical trials. Schizophr Res 111(1–3):39–45, 2009c 19375893
Colton CW, Manderscheid RW: Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis 3(2):A42, 2006 16539783
Crespo-Facorro B, Ortiz-García de la Foz V, Mata I, et al: Aripiprazole, Ziprasidone and Quetiapine in the treatment of first-episode nonaffective psychosis: a 12-week randomized, flexible-dose, open-label trial. Schizophr Res 147(2–3):375–382, 2013 23643328
Crespo-Facorro B, de la Foz VO, Mata I, et al: Treatment of first-episode non-affective psychosis: a randomized comparison of aripiprazole, quetiapine and ziprasidone over 1 year. Psychopharmacology (Berl) 231(2):357–366, 2014 23958945
Daniel DG: Tolerability of ziprasidone: an expanding perspective. J Clin Psychiatry 64 (suppl 19):40–49, 2003 14728089
Daniel DG, Zimbroff DL, Potkin SG, et al; Ziprasidone Study Group: Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Neuropsychopharmacology 20(5):491–505, 1999 10192829
Daniel DG, Potkin SG, Reeves KR, et al: Intramuscular (IM) ziprasidone 20 mg is effective in reducing acute agitation associated with psychosis: a double-blind, randomized trial. Psychopharmacology (Berl) 155(2):128–134, 2001 11401000
Davis JM, Chen N, Glick ID: A meta-analysis of the efficacy of second-generation antipsychotics. Arch Gen Psychiatry 60(6): 553–564, 2003 12796218
DelBello MP, Versavel M, Ice K, et al: Tolerability of oral ziprasidone in children and adolescents with bipolar mania, schizophrenia, or schizoaffective disorder. J Child Adolesc Psychopharmacol 18(5):491–499, 2008 18928413
DeLeon A, Patel NC, Crismon ML: Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clin Ther 26(5):649–666, 2004 15220010
Dew RE, Hughes D: Acute dystonic reaction with moderate-dose ziprasidone. J Clin Psychopharmacol 24(5):563–564, 2004 15349021
Díaz-Marsá M, Sánchez S, Rico-Villademoros F; ZIP-IIG-79 Study Group: Effectiveness and tolerability of oral ziprasidone in psychiatric inpatients with an acute exacerbation of schizophrenia or schizoaffective disorder: a multicenter, prospective, and naturalistic study. J Clin Psychiatry 70(4):509–517, 2009 19358789
Díaz-Mataix L, Scorza MC, Bortolozzi A, et al: Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity: role in atypical antipsychotic action. J Neurosci 25(47):10831–10843, 2005 16306396
Druss BG, Rosenheck RA: Mental disorders and access to medical care in the United States. Am J Psychiatry 155(12):1775–1777, 1998 9842793
Druss BG, Bradford DW, Rosenheck RA, et al: Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA 283(4):506–511, 2000 10659877
Druss BG, Bradford WD, Rosenheck RA, et al: Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry 58(6):565–572, 2001 11386985
Duggal HS: Ziprasidone-induced acute laryngeal dystonia. Prog Neuropsychopharmacol Biol Psychiatry 31(4):970; author reply 971, 2007 17343967
Duman RS: Depression: a case of neuronal life and death? Biol Psychiatry 56(3):140–145, 2004 15271581
Dwyer DS, Pinkofsky HB, Liu Y, et al: Antipsychotic drugs affect glucose uptake and the expression of glucose transporters in PC12 cells. Prog Neuropsychopharmacol Biol Psychiatry 23(1):69–80, 1999 10368857
Eli Lilly: Zyprexa (olanzapine tablets), Zyprexa (intramuscular olanzapine for injection), and Zyprexa Zydis (olanzapine orally disintegrating tablets), full prescribing information. August 2008. Available at: http://pi.lilly.com/us/zyprexa-pi.pdf. Accessed May 11, 2016.
Essock SM, Covell NH, Davis SM, et al: Effectiveness of switching antipsychotic medications. Am J Psychiatry 163(12):2090–2095, 2006 17151159
Everson G, Lasseter KC, Anderson KE, et al: The pharmacokinetics of ziprasidone in subjects with normal and impaired hepatic function. Br J Clin Pharmacol 49 (suppl 1):21S–26S, 2000 10771450
Farber NB, Rubin EH, Newcomer JW, et al: Increased neocortical neurofibrillary tangle density in subjects with Alzheimer disease and psychosis. Arch Gen Psychiatry 57(12):1165–1173, 2000 11115331
Findling RL, Cavus I, Pappadopulos E, et al: Efficacy, long-term safety, and tolerability of ziprasidone in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol 23(8):545–557, 2013a 24111980
Findling RL, Cavus I, Pappadopulos E, et al: Ziprasidone in adolescents with schizophrenia: results from a placebo-controlled efficacy and long-term open-extension study. J Child Adolesc Psychopharmacol 23(8):531–544, 2013b 24111983
Fontaine KR, Heo M, Harrigan EP, et al: Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 101(3):277–288, 2001 11311931
Frayne SM, Halanych JH, Miller DR, et al: Disparities in diabetes care: impact of mental illness. Arch Intern Med 165(22):2631–2638, 2005 16344421
Gandelman K, Alderman JA, Glue P, et al: The impact of calories and fat content of meals on oral ziprasidone absorption: a randomized, open-label, crossover trial. J Clin Psychiatry 70(1):58–62, 2009 19026256
Gao K, Pappadopulos E, Karayal ON, et al: Risk for adverse events and discontinuation due to adverse events of ziprasidone monotherapy relative to placebo in the acute treatment of bipolar depression, mania, and schizophrenia. J Clin Psychopharmacol 33(3):425–431, 2013 23609405
Garver DL, Holcomb JA, Christensen JD: Cerebral cortical gray expansion associated with two second-generation antipsychotics. Biol Psychiatry 58(1):62–66, 2005 15992524
Geddes J, Freemantle N, Harrison P, et al: Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ 321(7273):1371–1376, 2000 11099280
Gerhard T, Huybrechts K, Olfson M, et al: Comparative mortality risks of antipsychotic medications in community-dwelling older adults. Br J Psychiatry 205(1):44–51, 2014 23929443
Glassman AH, Bigger JT Jr: Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry 158(11):1774–1782, 2001 11691681
Glick ID, Romano SJ, Simpson G, et al: Insulin resistance in olanzapine- and ziprasidone-treated patients: results of a double-blind, controlled 6-week trial. Paper presented at the annual meeting of the American Psychiatric Association, New Orleans, LA, May 5–10, 2001
Goff DC, Posever T, Herz L, et al: An exploratory haloperidol-controlled dose-finding study of ziprasidone in hospitalized patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol 18(4):296–304, 1998 9690695
Goff DC, McEvoy JP, Citrome L, et al: High-dose oral ziprasidone versus conventional dosing in schizophrenia patients with residual symptoms: the ZEBRAS study. J Clin Psychopharmacol 33(4):485–490, 2013 23775057
Goldberg RJ, Bengtson J, Chen ZY, et al: Duration of the QT interval and total and cardiovascular mortality in healthy persons (The Framingham Heart Study experience). Am J Cardiol 67(1):55–58, 1991 1986505
Gómez-Criado MS, Bernardo M, Florez TD, et al: Ziprasidone overdose: cases recorded in the database of Pfizer-Spain and literature review. Pharmacotherapy 25(11):1660–1665, 2005 16232029
Gury C, Canceil O, Iaria P: [Antipsychotic drugs and cardiovascular safety: current studies of prolonged QT interval and risk of ventricular arrhythmia] (in French). Encephale 26(6):62–72, 2000 11217540
Hamelin BA, Allard S, Laplante L, et al: The effect of timing of a standard meal on the pharmacokinetics and pharmacodynamics of the novel atypical antipsychotic agent ziprasidone. Pharmacotherapy 18(1):9–15, 1998 9469675
Hardy TA, Poole-Hoffmann V, Lu Y, et al: Fasting glucose and lipid changes in patients with schizophrenia treated with olanzapine or ziprasidone. Poster presented at the annual meeting of the American College of Neuropsychopharmacology, San Juan, PR, December 7–11, 2003
Harrigan EP, Miceli JJ, Anziano R, et al: A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol 24(1):62–69, 2004 14709949
Harris EC, Barraclough B: Excess mortality of mental disorder. Br J Psychiatry 173:11–53, 1998 9850203
Harvey PD, Bowie CR: Ziprasidone: efficacy, tolerability, and emerging data on wide-ranging effectiveness. Expert Opin Pharmacother 6(2):337–346, 2005 15757429
Harvey PD, Siu CO, Romano S: Randomized, controlled, double-blind, multicenter comparison of the cognitive effects of ziprasidone versus olanzapine in acutely ill inpatients with schizophrenia or schizoaffective disorder. Psychopharmacology (Berl) 172(3):324–332, 2004 14615877
Harvey PD, Bowie CR, Loebel A: Neuropsychological normalization with long-term atypical antipsychotic treatment: results of a six-month randomized, double-blind comparison of ziprasidone vs. olanzapine. J Neuropsychiatry Clin Neurosci 18(1):54–63, 2006a 16525071
Harvey PD, Green MF, Bowie C, et al: The dimensions of clinical and cognitive change in schizophrenia: evidence for independence of improvements. Psychopharmacology (Berl) 187(3):356–363, 2006b 16783539
Harvey PD, Pappadopulos E, Lombardo I, et al: Reduction of functional disability with atypical antipsychotic treatment: a randomized long term comparison of ziprasidone and haloperidol. Schizophr Res 115(1):24–29, 2009 19189878
Hasnain M, Vieweg WV: QTc interval prolongation and torsade de pointes associated with second-generation antipsychotics and antidepressants: a comprehensive review. CNS Drugs 28(10):887–920, 2014 25168784
Haupt DW, Fahnestock PA, Flavin KA, et al: Adiposity and insulin sensitivity derived from intravenous glucose tolerance tests in antipsychotic-treated patients. Neuropsychopharmacology 32(12):2561–2569, 2007 17375138
Heinrich TW, Biblo LA, Schneider J: Torsades de pointes associated with ziprasidone. Psychosomatics 47(3):264–268, 2006 16684946
Heinrichs DW, Hanlon TE, Carpenter WT Jr: The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull 10(3):388–398, 1984 6474101
Hennekens CH, Hennekens AR, Hollar D, et al: Schizophrenia and increased risks of cardiovascular disease. Am Heart J 150(6):1115–1121, 2005 16338246
Hippisley-Cox J, Parker C, Coupland CA, et al: Inequalities in the primary care of patients with coronary heart disease and serious mental health problems: a cross-sectional study. Heart 93(10):1256–1262, 2007 17344333
Hirsch SR, Kissling W, Bäuml J, et al: A 28-week comparison of ziprasidone and haloperidol in outpatients with stable schizophrenia. J Clin Psychiatry 63(6):516–523, 2002 12088164
Hirschfeld RM, Keck PE Jr, Kramer M, et al: Rapid antimanic effect of risperidone monotherapy: a 3-week multicenter, double-blind, placebo-controlled trial. Am J Psychiatry 161(6):1057–1065, 2004 15169694
Holdridge KC, Sorsaburu S, Houston JP, et al: Characteristics and mortality among hospitalized patients treated with intramuscular antipsychotics: analysis of a United States hospital database. Curr Drug Saf 5(3):203–211, 2010 20210728
Houseknecht KL, Robertson AS, Zavadoski W, et al: Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology 32(2):289–297, 2007 17035934
Ichikawa J, Ishii H, Bonaccorso S, et al: 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem 76(5):1521–1531, 2001 11238736
Insa Gómez FJ, Gutiérrez Casares JR: Ziprasidone overdose: cardiac safety. Actas Esp Psiquiatr 33(6):398–400, 2005 16292724
Jarema M: Atypical antipsychotics in the treatment of mood disorders. Curr Opin Psychiatry 20(1):23–29, 2007 17143078
Jensen KG, Juul K, Fink-Jensen A, et al: Corrected QT changes during antipsychotic treatment of children and adolescents: a systematic review and meta-analysis of clinical trials. J Am Acad Child Adolesc Psychiatry 54(1):25–36, 2015 25524787
Johnsen E, Kroken RA, Wentzel-Larsen T, et al: Effectiveness of second-generation antipsychotics: a naturalistic, randomized comparison of olanzapine, quetiapine, risperidone, and ziprasidone. BMC Psychiatry 10:26, 2010 20334680
Joukamaa M, Heliövaara M, Knekt P, et al: Mental disorders and cause-specific mortality. Br J Psychiatry 179:498–502, 2001 11731351
Joyce AT, Harrison DJ, Loebel AD, et al: Effect of initial ziprasidone dose on length of therapy in schizophrenia. Schizophr Res 83(2–3):285–292, 2006 16545543
Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group: Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 371(9618):1085–1097, 2008 18374841
Kane JM: Introduction. Ziprasidone in schizophrenia: from acute treatment to long-term management. J Clin Psychiatry 64 (suppl 19):3–5, 2003a 14728083
Kane JM: Oral ziprasidone in the treatment of schizophrenia: a review of short-term trials. J Clin Psychiatry 64 (suppl 19):19–25, 2003b 14728086
Kane J, Khanna S, Rajadhyaksha S, Giller E: Efficacy and tolerability of ziprasidone in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol 21(1):21–28, 2006 16317313
Kapur S, Remington G: Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry 153(4):466–476, 1996 8599393
Kapur S, Remington G: Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry 50(11): 873–883, 2001 11743942
Kaufman KR, Stern L, Mohebati A, et al: Ziprasidone-induced priapism requiring surgical treatment. Eur Psychiatry 21(1):48–50, 2006 16356688
Keck ME, Müller MB, Binder EB, et al: Ziprasidone-related tardive dyskinesia. Am J Psychiatry 161(1):175–176, 2004 14702272
Keck P Jr, Buffenstein A, Ferguson J, et al: Ziprasidone 40 and 120 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 4-week placebo-controlled trial. Psychopharmacology (Berl) 140(2):173–184, 1998 9860108
Keck PE Jr, Reeves KR, Harrigan EP; Ziprasidone Study Group: Ziprasidone in the short-term treatment of patients with schizoaffective disorder: results from two double-blind, placebo-controlled, multicenter studies. J Clin Psychopharmacol 21(1):27–35, 2001 11199944
Keck PE Jr, Marcus R, Tourkodimitris S, et al; Aripiprazole Study Group: A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 160(9):1651–1658, 2003a 12944341
Keck PE Jr, Versiani M, Potkin S, et al; Ziprasidone in Mania Study Group: Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trial. Am J Psychiatry 160(4):741–748, 2003b 12668364
Keck PE Jr, Potkin S, Warrington L, et al: Efficacy and safety of ziprasidone in bipolar disorder: short- and long-term data. Poster presented at the annual meeting of the American Psychiatric Association, New York, May 1–6, 2004
Keck PE Jr, Versiani M, Warrington L, et al: Long-term safety and efficacy of ziprasidone in subpopulations of patients with bipolar mania. J Clin Psychiatry 70(6): 844–851, 2009 19573482
Keefe RS, Bilder RM, Davis SM, et al; CATIE Investigators; Neurocognitive Working Group: Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry 64(6):633–647, 2007 17548746
Khanna S, Vieta E, Lyons B, et al: Risperidone in the treatment of acute mania: double-blind, placebo-controlled study. Br J Psychiatry 187:229–234, 2005 16135859
Kinon BJ, Lipkovich I, Edwards SB, et al: A 24-week randomized study of olanzapine versus ziprasidone in the treatment of schizophrenia or schizoaffective disorder in patients with prominent depressive symptoms. J Clin Psychopharmacol 26(2):157–162, 2006 16633144
Koro CE, Fedder DO, L’Italien GJ, et al: An assessment of the independent effects of olanzapine and risperidone exposure on the risk of hyperlipidemia in schizophrenic patients. Arch Gen Psychiatry 59(11):1021–1026, 2002a 12418935
Koro CE, Fedder DO, L’Italien GJ, et al: Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study. BMJ 325(7358):243, 2002b 12153919
Kreyenbuhl J, Marcus SC, West JC, et al: Adding or switching antipsychotic medications in treatment-refractory schizophrenia. Psychiatr Serv 58(7):983–990, 2007 17602016
Kroeze WK, Hufeisen SJ, Popadak BA, et al: H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 28(3):519–526, 2003 12629531
Kudla D, Lambert M, Domin S, et al: Effectiveness, tolerability, and safety of ziprasidone in patients with schizophrenia or schizoaffective disorder: results of a multi-centre observational trial. Eur Psychiatry 22(3):195–202, 2007 17140769
Kuehn BM: FDA panel OKs 3 antipsychotic drugs for pediatric use, cautions against overuse. JAMA 302(8):833–834, 2009 19706851
Lesem MD, Zajecka JM, Swift RH, et al: Intramuscular ziprasidone, 2 mg versus 10 mg, in the short-term management of agitated psychotic patients. J Clin Psychiatry 62(1):12–18, 2001 11235922
Leucht S, Pitschel-Walz G, Abraham D, et al: Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr Res 35(1):51–68, 1999 9988841
Leucht S, Cipriani A, Spineli L, et al: Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382(9896):951–962, 2013 23810019
Levy WO, Robichaux-Keene NR, Nunez C: No significant QTc interval changes with high-dose ziprasidone: a case series. J Psychiatr Pract 10(4):227–232, 2004 15552544
Lieberman JA: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia: efficacy, safety and cost outcomes of CATIE and other trials. J Clin Psychiatry 68(2):e04, 2007 17335312
Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353(12):1209–1223, 2005 16172203
Liebowitz MR, Salmán E, Mech A, et al: Ziprasidone monotherapy in bipolar II depression: an open trial. J Affect Disord 118(1–3):205–208, 2009 19264363
Loebel A, Siu C, Romano S: Improvement in prosocial functioning after a switch to ziprasidone treatment. CNS Spectr 9(5):357–364, 2004 15115948
Lombardo I, Alderman J, Preskorn S, et al: Effect of food on absorption of ziprasidone (abstract of poster presented at the International Congress on Schizophrenia Research, March 28–April 1, 2007, Colorado Springs, CO). Schizophr Bull 33(2):475–476, 2007
Mamo D, Kapur S, Shammi CM, et al: A PET study of dopamine D2 and serotonin 5-HT2 receptor occupancy in patients with schizophrenia treated with therapeutic doses of ziprasidone. Am J Psychiatry 161(5):818–825, 2004 15121646
Mason MN, Johnson CE, Piasecki M: Ziprasidone-induced acute dystonia. Am J Psychiatry 162(3):625–626, 2005 15741487
Mazei MS, Pluto CP, Kirkbride B, et al: Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res 936(1–2):58–67, 2002 11988230
McCue RE, Waheed R, Urcuyo L, et al: Comparative effectiveness of second-generation antipsychotics and haloperidol in acute schizophrenia. Br J Psychiatry 189: 433–440, 2006 17077434
McEvoy JP, Meyer JM, Goff DC, et al: Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 80(1):19–32, 2005 16137860
McEvoy JP, Lieberman JA, Perkins DO, et al: Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry 164(7):1050–1060, 2007 17606657
McIntyre RS, Brecher M, Paulsson B, et al: Quetiapine or haloperidol as monotherapy for bipolar mania—a 12-week, double-blind, randomised, parallel-group, placebo-controlled trial. Eur Neuropsychopharmacol 15(5):573–585, 2005 16139175
McQuade RD, Marcus R, Sanchez R: Aripiprazole vs placebo in acute mania: safety and tolerability pooled analysis. Poster presented at the International Conference on Bipolar Disorder, Pittsburgh, PA, June 12–14, 2003
Miceli JJ, Anziano RJ, Robarge L, et al: The effect of carbamazepine on the steady-state pharmacokinetics of ziprasidone in healthy volunteers. Br J Clin Pharmacol 49 (suppl 1):65S–70S, 2000a 10771457
Miceli JJ, Smith M, Robarge L, et al: The effects of ketoconazole on ziprasidone pharmacokinetics—a placebo-controlled crossover study in healthy volunteers. Br J Clin Pharmacol 49 (suppl 1):71S–76S, 2000b 10771458
Miceli JJ, Wilner KD, Hansen RA, et al: Single- and multiple-dose pharmacokinetics of ziprasidone under non-fasting conditions in healthy male volunteers. Br J Clin Pharmacol 49 (suppl 1):5S–13S, 2000c 10771448
Miceli JJ, Murray S, Sallee FR, et al: Pharmacokinetic and pharmacodynamic QTc profile of oral ziprasidone in pediatric and adult subjects following single-dose administration. Poster presented at the annual meeting of the American Psychiatric Association, New York, May 1–6, 2004
Miceli JJ, Tensfeldt TG, Shiovitz T, et al: Effects of high-dose ziprasidone and haloperidol on the QTc interval after intramuscular administration: a randomized, single-blind, parallel-group study in patients with schizophrenia or schizoaffective disorder. Clin Ther 32(3):472–491, 2010 20399985
Millan MJ: Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther 295(3): 853–861, 2000 11082417
Miodownik C, Hausmann M, Frolova K, et al: Lithium intoxication associated with intramuscular ziprasidone in schizoaffective patients. Clin Neuropharmacol 28(6):295–297, 2005 16340388
Montanez A, Ruskin JN, Hebert PR, et al: Prolonged QTc interval and risks of total and cardiovascular mortality and sudden death in the general population: a review and qualitative overview of the prospective cohort studies. Arch Intern Med 164(9):943–948, 2004 15136301
Montes JM, Rodriguez JL, Balbo E, et al: Improvement in antipsychotic-related metabolic disturbances in patients with schizophrenia switched to ziprasidone. Prog Neuropsychopharmacol Biol Psychiatry 31(2):383–388, 2007 17129654
Morrato EH, Newcomer JW, Allen RR, et al: Prevalence of baseline serum glucose and lipid testing in users of second-generation antipsychotic drugs: a retrospective, population-based study of Medicaid claims data. J Clin Psychiatry 69(2):316–322, 2008 18251625
Mullins CD, Shaya FT, Zito JM, et al: Effect of initial ziprasidone dose on treatment persistence in schizophrenia. Schizophr Res 83(2–3):277–284, 2006 16545945
Muralidharan K, Ali M, Silveira LE, et al: Efficacy of second generation antipsychotics in treating acute mixed episodes in bipolar disorder: a meta-analysis of placebo-controlled trials. J Affect Disord 150(2):408–414, 2013 23735211
Murray S, Mandel FS, Loebel A: Optimal initial dosing of ziprasidone: clinical trial data. Poster presented at the annual meeting of the American Psychiatric Association, New York, May 1–6, 2004
Murty RG, Mistry SG, Chacko RC: Neuroleptic malignant syndrome with ziprasidone. J Clin Psychopharmacol 22(6):624–626, 2002 12454565
Nasrallah HA, Meyer JM, Goff DC, et al: Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 86(1–3):15–22, 2006 16884895
Nemeroff CB, Lieberman JA, Weiden PJ, et al: From clinical research to clinical practice: a 4-year review of ziprasidone. CNS Spectr 10 (11 suppl 17):1–20, 2005 16381088
Newcomer JW: Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 19 (suppl 1):1–93, 2005 15998156
Newcomer JW, Haupt DW: The metabolic effects of antipsychotic medications. Can J Psychiatry 51(8):480–491, 2006 16933585
Newcomer JW, Hennekens CH: Severe mental illness and risk of cardiovascular disease. JAMA 298(15):1794–1796, 2007 17940236
Obach RS, Huynh P, Allen MC, et al: Human liver aldehyde oxidase: inhibition by 239 drugs. J Clin Pharmacol 44(1):7–19, 2004 14681337
O’Brien JM, Rockwood RP, Suh KI: Haloperidol-induced torsade de pointes. Ann Pharmacother 33(10):1046–1050, 1999 10534216
O’Connor R, Schooler NR: Penultimate observation carried forward (POCF): a new approach to analysis of long-term symptom change in chronic relapsing conditions. Schizophr Res 60(2–3):319–320, 2003 12591593
Olfson M, Gerhard T, Huang C, et al: Comparative effectiveness of second-generation antipsychotic medications in early onset schizophrenia. Schizophr Bull 38(4):845–853, 2012 21307041
Osby U, Correia N, Brandt L, et al: Mortality and causes of death in schizophrenia in Stockholm county, Sweden. Schizophr Res 45(1–2):21–28, 2000 10978869
Osby U, Brandt L, Correia N, et al: Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 58(9):844–850, 2001 11545667
Papakostas GI, Petersen TJ, Nierenberg AA, et al: Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J Clin Psychiatry 65(2):217–221, 2004 15003076
Papakostas GI, Vitolo OV, Ishak WW, et al: A 12-week, randomized, double-blind, placebo-controlled, sequential parallel comparison trial of ziprasidone as monotherapy for major depressive disorder. J Clin Psychiatry 73(12):1541–1547, 2012 23290327
Papakostas GI, Fava M, Baer L, et al: Ziprasidone augmentation of escitalopram for major depressive disorder: Efficacy results from a randomized, double-blind, placebo-controlled study. Am J Psychiatry 172(12):1251–1258, 2015 26085041
Papapetropoulos S, Wheeler S, Singer C: Tardive dystonia associated with ziprasidone. Am J Psychiatry 162(11):2191, 2005 16263868
Patel NC, Keck PE Jr: Ziprasidone: efficacy and safety in patients with bipolar disorder. Expert Rev Neurother 6(8):1129–1138, 2006 16893341
Perlis RH, Welge JA, Vornik LA, et al: Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry 67(4):509–516, 2006 16669715
Pfizer Inc: Dear Healthcare Practitioner letter, August 2004. Available at: http://www.fda.gov/medwatch/SAFETY/2004/GeodonDearDoc.pdf. Accessed May 12, 2016.
Pfizer Inc: Geodon (ziprasidone HCl) capsules and Geodon (ziprasidone mesylate) for injection, full prescribing information, June 2008
Potkin SG, Keck PE Jr, Segal S, et al: Ziprasidone in acute bipolar mania: a 21-day randomized, double-blind, placebo-controlled replication trial. J Clin Psychopharmacol 25(4):301–310, 2005 16012271
Potkin SG, Weiden PJ, Loebel AD, et al: Remission in schizophrenia: 196-week, double-blind treatment with ziprasidone vs. haloperidol. Int J Neuropsychopharmacol 12(9):1233–1248, 2009 19419595
Pozzi L, Acconcia S, Ceglia I, et al: Stimulation of 5-hydroxytryptamine (5-HT(2C)) receptors in the ventrotegmental area inhibits stress-induced but not basal dopamine release in the rat prefrontal cortex. J Neurochem 82(1):93–100, 2002 12091469
Prakash C, Kamel A, Cui D, et al: Identification of the major human liver cytochrome P450 isoform(s) responsible for the formation of the primary metabolites of ziprasidone and prediction of possible drug interactions. Br J Clin Pharmacol 49 (suppl 1):35S–42S, 2000 10771452
Purnine DM, Carey KB, Maisto SA, et al: Assessing positive and negative symptoms in outpatients with schizophrenia and mood disorders. J Nerv Ment Dis 188(10): 653–661, 2000 11048814
Ramos AE, Shytle RD, Silver AA, et al: Ziprasidone-induced oculogyric crisis. J Am Acad Child Adolesc Psychiatry 42(9): 1013–1014, 2003 12964566
Rettenbacher MA, Ebenbichler C, Hofer A, et al: Early changes of plasma lipids during treatment with atypical antipsychotics. Int Clin Psychopharmacol 21(6):369–372, 2006 17012984
Rochon PA, Normand SL, Gomes T, et al: Antipsychotic therapy and short-term serious events in older adults with dementia. Arch Intern Med 168(10):1090–1096, 2008 18504337
Rollema H, Lu Y, Schmidt AW, et al: 5-HT(1A) receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biol Psychiatry 48(3):229–237, 2000 10924666
Rosenfield PJ, Girgis RR, Gil R: High-dose ziprasidone-induced acute dystonia. Prog Neuropsychopharmacol Biol Psychiatry 31(2):546–547, 2007 17123682
Rossi A, Cañas F, Fagiolini A, et al: Switching among antipsychotics in everyday clinical practice: focus on ziprasidone. Postgrad Med 123(1):135–159, 2011 21293094
Rummel-Kluge C, Komossa K, Schwarz S, et al: Second-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisons. Schizophr Bull 38(1):167–177, 2012 20513652
Rybakowski JK, Vansteelandt K, Remlinger-Molenda A, et al; EUFEST Study Group: Extrapyramidal symptoms during treatment of first schizophrenia episode: results from EUFEST. Eur Neuropsychopharmacol 24(9):1500–1505, 2014 25085534
Ryu S, Yoo JH, Kim JH, et al: Tardive dyskinesia and tardive dystonia with second-generation antipsychotics in non-elderly schizophrenic patients unexposed to first-generation antipsychotics: a cross-sectional and retrospective study. J Clin Psychopharmacol 35(1):13–21, 2015 25485636
Sacchetti E, Galluzzo A, Valsecchi P, et al; MOZART Study Group: Ziprasidone vs clozapine in schizophrenia patients refractory to multiple antipsychotic treatments: the MOZART study. Schizophr Res 113(1):112–121, 2009 19606529
Sachs G, Sanchez R, Marcus R, et al; Aripiprazole Study Group: Aripiprazole in the treatment of acute manic or mixed episodes in patients with bipolar I disorder: a 3-week placebo-controlled study. J Psychopharmacol 20(4):536–546, 2006 16401666
Scherk H, Pajonk FG, Leucht S: Second-generation antipsychotic agents in the treatment of acute mania: a systematic review and meta-analysis of randomized controlled trials. Arch Gen Psychiatry 64(4):442–455, 2007 17404121
Schizophrenia.com: Eli Lilly updates label warning for Zyprexa to better inform on side-effects. October 5, 2007. Available at: http://www.schizophrenia.com/sznews/archives/005617.html. Accessed May 11, 2016.
Schmidt AW, Lebel LA, Howard HR Jr, et al: Ziprasidone: a novel antipsychotic agent with a unique human receptor binding profile. Eur J Pharmacol 425(3):197–201, 2001 11513838
Schooler NR: Maintaining symptom control: review of ziprasidone long-term efficacy data. J Clin Psychiatry 64 (suppl 19):26–32, 2003 14728087
Seeger TF, Seymour PA, Schmidt AW, et al: Ziprasidone (CP-88,059): a new antipsychotic with combined dopamine and serotonin receptor antagonist activity. J Pharmacol Exp Ther 275(1):101–113, 1995 7562537
Simpson G, Weiden P, Pigott TA, et al: Ziprasidone vs olanzapine in schizophrenia: 6-month continuation study (abstract). Eur Neuropsychopharmacol 12 (suppl 3): S310, 2002
Simpson GM, Glick ID, Weiden PJ, et al: Randomized, controlled, double-blind multicenter comparison of the efficacy and tolerability of ziprasidone and olanzapine in acutely ill inpatients with schizophrenia or schizoaffective disorder. Am J Psychiatry 161(10):1837–1847, 2004a 15465981
Simpson GM, Weiden PJ, Loebel A, et al: Ziprasidone: long-term post-switch efficacy in schizophrenia. Poster presented at the annual meeting of the American Psychiatric Association, New York, May 1–6, 2004b
Simpson GM, Weiden P, Pigott T, et al: Six-month, blinded, multicenter continuation study of ziprasidone versus olanzapine in schizophrenia. Am J Psychiatry 162(8):1535–1538, 2005 16055779
Smulevich AB, Khanna S, Eerdekens M, et al: Acute and continuation risperidone monotherapy in bipolar mania: a 3-week placebo-controlled trial followed by a 9-week double-blind trial of risperidone and haloperidol. Eur Neuropsychopharmacol 15(1):75–84, 2005 15572276
Srisurapanont M, Maneeton N: Comparison of the efficacy and acceptability of atypical antipsychotic drugs: a meta-analysis of randomized, placebo-controlled trials. J Med Assoc Thai 82(4):341–346, 1999 10410494
Stahl SM: Neurotransmission of cognition, part 2. Selective NRIs are smart drugs: exploiting regionally selective actions on both dopamine and norepinephrine to enhance cognition. J Clin Psychiatry 64(2):110–111, 2003 12633117
Stahl SM, Shayegan DK: The psychopharmacology of ziprasidone: receptor-binding properties and real-world psychiatric practice. J Clin Psychiatry 64 (suppl 19):6–12, 2003 14728084
Stahl S, Lombardo I, Loebel A, et al: Efficacy of ziprasidone in dysphoric mania: pooled analysis of two double-blind studies. J Affect Disord 122(1–2):39–45, 2010a 19616304
Stahl SM, Malla A, Newcomer JW, et al: A post hoc analysis of negative symptoms and psychosocial function in patients with schizophrenia: a 40-week randomized, double-blind study of ziprasidone versus haloperidol followed by a 3-year double-blind extension trial. J Clin Psychopharmacol 30(4):425–430, 2010b 20571437
Strassnig M, Clarke J, Mann S, et al: Body composition, pre-diabetes and cardiovascular disease risk in early schizophrenia. Early Interv Psychiatry March 10, 2015 [Epub ahead of print] 25752319
Strom BL, Eng SM, Faich G, et al: Comparative mortality associated with ziprasidone and olanzapine in real-world use among 18,154 patients with schizophrenia: The Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC). Am J Psychiatry 168(2):193–201, 2011 21041245
Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators: Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 163(4):611–622, 2006 16585435
Sumiyoshi T, Jayathilake K, Meltzer HY: The effect of melperone, an atypical antipsychotic drug, on cognitive function in schizophrenia. Schizophr Res 59(1):7–16, 2003 12413636
Swainston Harrison T, Scott LJ: Ziprasidone: a review of its use in schizophrenia and schizoaffective disorder. CNS Drugs 20(12):1027–1052, 2006 17140281
Swartz MS, Perkins DO, Stroup TS, et al; CATIE Investigators: Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. Am J Psychiatry 164(3):428–436, 2007 17329467
Swift RH, Harrigan EP, van Kammen DP: A comparison of fixed-dose intramuscular (IM) ziprasidone with flexible-dose IM haloperidol. Poster presented at the annual meeting of the American Psychiatric Association, Toronto, ON, Canada, May 30–June 4, 1998
Tan HH, Hoppe J, Heard K: A systematic review of cardiovascular effects after atypical antipsychotic medication overdose. Am J Emerg Med 27(5):607–616, 2009 19497468
Tandon R, Fleischhacker WW: Comparative efficacy of antipsychotics in the treatment of schizophrenia: a critical assessment. Schizophr Res 79(2–3):145–155, 2005 16139989
Tatsumi M, Jansen K, Blakely RD, et al: Pharmacological profile of neuroleptics at human monoamine transporters. Eur J Pharmacol 368(2–3):277–283, 1999 10193665
Tauscher J, Kapur S, Verhoeff NP, et al: Brain serotonin 5-HT(1A) receptor binding in schizophrenia measured by positron emission tomography and [11C]WAY-100635. Arch Gen Psychiatry 59(6):514–520, 2002 12044193
Taylor D: Ziprasidone in the management of schizophrenia: the QT interval issue in context. CNS Drugs 17(6):423–430, 2003 12697001
Tecott LH, Sun LM, Akana SF, et al: Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 374(6522):542–546, 1995 7700379
Thome J, Foley P, Riederer P: Neurotrophic factors and the maldevelopmental hypothesis of schizophrenic psychoses. Review article. J Neural Transm (Vienna) 105(1):85–100, 1998 9588763
Tohen M, Sanger TM, McElroy SL, et al; Olanzapine HGEH Study Group: Olanzapine versus placebo in the treatment of acute mania. Am J Psychiatry 156(5):702–709, 1999 10327902
Tohen M, Jacobs TG, Grundy SL, et al; The Olanzipine HGGW Study Group: Efficacy of olanzapine in acute bipolar mania: a double-blind, placebo-controlled study. Arch Gen Psychiatry 57(9):841–849, 2000 10986547
Versavel M, DelBello MP, Ice K, et al: Ziprasidone dosing study in pediatric patients with bipolar disorder, schizophrenia, or schizoaffective disorder (abstract). Neuropsychopharmacology 30 (suppl 1):S122–S123, 2005
Vieta E, Ramey T, Keller D, et al: Ziprasidone in the treatment of acute mania: a 12-week, placebo-controlled, haloperidol-referenced study. J Psychopharmacol 24(4):547–558, 2010 19074536
Villanueva N, Markham-Abedi C, McNeely C, et al: Probable association between ziprasidone and worsening hypertension. Pharmacotherapy 26(9):1352–1357, 2006 16945059
Weiden PJ, Iqbal N, Mendelowitz AJ, et al: Best clinical practice with ziprasidone: update after one year of experience. J Psychiatr Pract 8(2):81–97, 2002 15985861
Weiden PJ, Daniel DG, Simpson G, et al: Improvement in indices of health status in outpatients with schizophrenia switched to ziprasidone. J Clin Psychopharmacol 23(6):595–600, 2003a 14624190
Weiden PJ, Simpson GM, Potkin SG, et al: Effectiveness of switching to ziprasidone for stable but symptomatic outpatients with schizophrenia. J Clin Psychiatry 64(5):580–588, 2003b 12755663
Weiden PJ, Newcomer JW, Loebel AD, et al: Long-term changes in weight and plasma lipids during maintenance treatment with ziprasidone. Neuropsychopharmacology 33(5):985–994, 2008 17637612
Weinstein SK, Adler CM, Strakowski SM: Ziprasidone-induced acute dystonic reactions in patients with bipolar disorder. J Clin Psychiatry 67(2):327–328, 2006 16566635
Weisler R, Dunn J, English P: Ziprasidone in adjunctive treatment of acute bipolar mania: double-blind, placebo-controlled trial. Poster presented at the annual meeting of the Institute on Psychiatric Services, Boston, MA, October 29–November 2, 2003
Weisler R, Warrington L, Dunn J: Adjunctive ziprasidone in bipolar mania: short- and long-term data (abstract). Biol Psychiatry 55 (8 suppl):43S, 2004
Yumru M, Savas HA, Selek S, et al: Acute dystonia after initial doses of ziprasidone: a case report. Prog Neuropsychopharmacol Biol Psychiatry 30(4):745–747, 2006 16580766
Ziegenbein M, Schomerus G, Kropp S: Ziprasidone-induced Pisa syndrome after clozapine treatment. J Neuropsychiatry Clin Neurosci 15(4):458–459, 2003 14627778
Zimbroff DL, Brook S, Benattia I: Safety and tolerability of IM ziprasidone: review of clinical trial data. Poster presented at the annual meeting of the American Psychiatric Association, Philadelphia, PA, May 18–23, 2002
Zink M, Kuwilsky A, Krumm B, et al: Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomised controlled clinical trial. J Psychopharmacol 23(3):305–314, 2009 18562423
Zhang H, Wang G, Zhao J, et al: Intramuscular ziprasidone versus haloperidol for managing agitation in Chinese patients with schizophrenia. J Clin Psychopharmacol 33(2):178–185, 2013 23422376
Zorn SH, Bebel LA, Schmidt AW, et al: Pharmacological and neurochemical studies with the new antipsychotic ziprasidone, in Interactive Monoaminergic Basis of Brain Disorders. Edited by Palomo T, Beninger R, Archer T. Madrid, Spain, Editorial Sintesis, 1998, pp 377–394