 Patients with cancer are taking antioxidant supplements in combination with conventional chemotherapy and radiotherapy to enhance the anticancer activity and to reduce the side effects of conventional treatment.
Patients with cancer are taking antioxidant supplements in combination with conventional chemotherapy and radiotherapy to enhance the anticancer activity and to reduce the side effects of conventional treatment.KEY CONCEPTS
 Patients with cancer are taking antioxidant supplements in combination with conventional chemotherapy and radiotherapy to enhance the anticancer activity and to reduce the side effects of conventional treatment.
Patients with cancer are taking antioxidant supplements in combination with conventional chemotherapy and radiotherapy to enhance the anticancer activity and to reduce the side effects of conventional treatment.
 Many oncologists are concerned that the protective mechanisms of antioxidants may not distinguish between normal and malignant cells, so that supplements may interfere with the anticancer activity of the conventional therapies.
Many oncologists are concerned that the protective mechanisms of antioxidants may not distinguish between normal and malignant cells, so that supplements may interfere with the anticancer activity of the conventional therapies.
 Not all antioxidant supplements are equivalent; antioxidants counteract free radical activity through multiple mechanisms of action.
Not all antioxidant supplements are equivalent; antioxidants counteract free radical activity through multiple mechanisms of action.
 Not all chemotherapy agents rely on oxidative stress for anticancer activity, so risk of interaction with antioxidant supplements is also dependent on the type of conventional chemotherapy.
Not all chemotherapy agents rely on oxidative stress for anticancer activity, so risk of interaction with antioxidant supplements is also dependent on the type of conventional chemotherapy.
 Several studies have shown that plasma concentrations of antioxidants are depleted in individuals undergoing treatment for cancer; some studies suggest that this decrease may be associated with therapy-related toxicities.
Several studies have shown that plasma concentrations of antioxidants are depleted in individuals undergoing treatment for cancer; some studies suggest that this decrease may be associated with therapy-related toxicities.
 Few randomized controlled trials have investigated the efficacy of antioxidants as a treatment for cancer, most of the trials published have been case series or nonrandomized trials.
Few randomized controlled trials have investigated the efficacy of antioxidants as a treatment for cancer, most of the trials published have been case series or nonrandomized trials.
 Most of the clinical trials published have described the use of antioxidants as supportive care agents; the results of these trials have been mixed.
Most of the clinical trials published have described the use of antioxidants as supportive care agents; the results of these trials have been mixed.
 Based on the findings from a large randomized controlled trial, the combined use of β-carotene and vitamin E supplements in conjunction with radiation therapy is contraindicated and should be avoided until further research is available.
Based on the findings from a large randomized controlled trial, the combined use of β-carotene and vitamin E supplements in conjunction with radiation therapy is contraindicated and should be avoided until further research is available.
 As few randomized controlled trials have addressed the use of antioxidant supplements during chemotherapy, health care providers should be cautious about recommending antioxidant supplements during chemotherapy until further research is available to guide clinical practice.
As few randomized controlled trials have addressed the use of antioxidant supplements during chemotherapy, health care providers should be cautious about recommending antioxidant supplements during chemotherapy until further research is available to guide clinical practice.

The use of antioxidant supplements by patients during conventional cancer treatment is among one of the most controversial areas in oncology. Past estimates of antioxidant use by patients with cancer have varied considerably, with rates ranging from 13%–87% depending on the survey, the type of cancer studied, and a variety of other individual and demographic factors (Block, et al., 2007; Branda, Naud, Brooks, Chen, & Muss, 2004; Burstein, Gelber, Guadagnoli, & Weeks, 1999; Clemens, Waladkhani, Bublitz, Ehninger, & Gey, 1997; Kelly, et al., 2000; Legha, et al., 1982; Lesperance, et al., 2002). In one large study, the Women’s Health Eating Initiative, 58% of women with breast cancer reported taking multivitamin supplements, 46% used Vitamin E, 42% took vitamin C and 10% supplemented with an antioxidant mixture (Rock, Newman, Neuhouser, Major, & Barnett, 2004). However, data is still lacking on the use of antioxidant supplements among patients with other types of cancer, and the doses used in conjunction with conventional therapies.
Antioxidants are substances that counteract free radicals and prevent them from causing tissue and organ damage (Ratnam, Ankola, Bhardwaj, Sahana, & Kumar, 2006). Evidence supporting the potential role of antioxidants in preventing and treating disease include preclinical studies, which have correlated oxidative stress and an antioxidant-depleted diet with the development of diseases including cancer (Salganik, et al., 2000). Some epidemiologic studies have observed an association between an increased intake of dietary antioxidants and a decreased risk of developing lung (Mannisto, et al., 2004; Yong, et al., 1997), esophageal (Mark, et al., 2000), and gastrointestinal (Bjelakovic, Nikolova, Simonetti, & Gluud, 2004; Jenab, et al., 2006a; Jenab, et al., 2006b) cancer. Antioxidants are most commonly taken in conjunction with conventional cancer treatment rather than as its replacement. At the current time, the precise role of antioxidant supplementation in the patient with established cancer remains to be determined.
Table 9.1. Oxidative Stress and Chemotherapy.
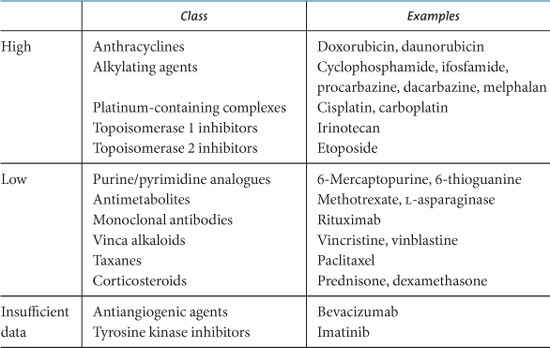
Chemotherapy agents vary in their risk for interaction with antioxidant supplements. Table 9.1 lists chemotherapy agents by association with the generation of oxidative stress, with “high oxidative stress” agents being at potentially increased risk for interaction with antioxidant supplements.
Much of the controversy surrounding antioxidants and cancer therapy has arisen because certain classes of chemotherapy agents exert some of their anticancer effects by generating reactive oxygen species, or free radicals (Moss, 2006). Some of these agents include the anthracyclines (e.g., doxorubicin), platinum-containing complexes (e.g., cisplatin, carboplatin), and alkylating agents (e.g., cyclophosphamide, ifosfamide), as well as radiation therapy. The theoretical concern is that antioxidants might somehow interfere with or counteract the activities of these anticancer agents. However, many chemotherapy agents have multiple mechanisms of action and do not necessarily rely on the generation of free radicals (Table 9.1).
Free radical–mediated damage to normal tissues often manifests as side effects of chemotherapy, such as anthracycline-associated cardiomyopathy, or hearing loss or kidney failure caused by platinum-based agents. Antioxidant supplementation may facilitate anticancer treatment by protecting normal tissues and allowing for higher doses of chemotherapy to be administered. Additionally, at certain concentrations, they might also be able to directly affect cancer cells through prooxidant effects.
Many proponents for combining antioxidant supplements with conventional cancer therapy justify this approach because observational studies have identified the depletion of antioxidant levels during treatment with radiation and chemotherapy, particularly with the use of conditioning regimens before stem cell transplantation (Durken, et al., 2000; Kennedy, Ladas, Rheingold, Blumberg, & Kelly, 2004a; Kennedy, et al., 2004b).
The use of accepted pharmaceutical protective agents that work through antioxidant mechanisms further supports the use of antioxidant supplements during chemotherapy. Mesna, for example, minimizes the risk of hemorrhagic cystitis by forming nontoxic compounds in the bladder with acrolein, 4-hydroxy-metabolites, and other urotoxic metabolites of oxazaphosphorines related to ifosfamide and cyclophosphamide metabolism. The thiol metabolite of amifostine is readily taken up by cells where it binds to and detoxifies reactive metabolites of platinum and alkylating agents as well as scavenges free radicals. Tumor cells are generally not protected because amifostine and metabolites are present in normal cells at 100-fold greater concentrations than in tumor cells (Creagan, et al., 1979; Culy & Spencer, 2001, 2002; McEvoy, 2002; Moertel, et al., 1985). The use of these cytoprotective agents is based on the results of preclinical studies and evidence accumulated from clinical trials to date, which is still lacking for most antioxidant supplements.
A limited number of clinical trials have investigated antioxidant supplementation for the treatment of specific cancers, or for the reduction in or prevention of common adverse effects associated with anticancer therapy (Bairati, et al., 2005; Drisko, Chapman, & Hunter, 2003a; Drisko, Chapman, & Hunter, 2003b; Hoenjet, et al., 2005; Ladas, et al., 2004; Pathak, et al., 2005). Several systematic reviews have evaluated the health advantages of using antioxidant supplements concomitantly with chemotherapy (Block, et al., 2007; Ladas, et al., 2004; Simone, Simone, Simone, & Simone, 2007a, 2007b). Most clinical trials have been limited by inadequate sample sizes, heterogeneous patient populations, variation in the routes of antioxidant administration, or study designs that lacked appropriate blinding to randomization (Ladas, et al., 2004). Although the data on antioxidant use during chemotherapy has not been associated with an attenuation of the effects of the particular anticancer treatment on tumor control, the studies have not yet thoroughly investigated this issue to allow for the routine recommendation of antioxidant supplementation. This chapter provides an overview of the issues surrounding this very controversial topic, with a summary of the key clinical trial evidence that has been reported to date.
Antioxidant is a broad term that refers to a myriad of different compounds. Because of the disparity in the biological actions and targets of antioxidants, there is no simple paradigm for advising patients of their safe use during conventional chemotherapy and radiotherapy. Antioxidants function through a variety of mechanisms and each may belong to more than one functional category (Papas, 1999).
There are two general categories of antioxidants:
• Enzymatic antioxidants such as catalase, superoxide dismutase, and glutathione peroxidase
• Nonenzymatic antioxidants, such as vitamin C, vitamin E, coenzyme Q10, melatonin, and pycnogenol
Within these two broad categories, there are four functional subclasses:
• Preventative agents that suppress the formation of free radicals
• Radical scavenging agents that inhibit chain initiation and/or propagation
• Repair and de novo enzymes that repair and reconstitute cell membranes
• Adaptation agents that generate appropriate antioxidant enzymes and transfer them to the site of action
The wide spectrum of activity of antioxidant compounds further complicates the counsel for patients on the safety of taking antioxidants in combination with conventional cancer therapy.
Most nonenzymatic antioxidant compounds or their precursors are obtained orally, either through foods or dietary supplements. Although dietary intake of antioxidant-rich foods has been shown to elevate human plasma antioxidant levels, it is unlikely that intake from the typical 2500-calorie American adult diet could effectively achieve the steady state levels required to interact with the chemotherapy concentrations used in anticancer treatments. Although one study in children being treated for acute lymphoblastic leukemia found that an increased dietary intake of antioxidant nutrients was associated with reductions in chemotherapy-associated side effects (Kennedy, et al., 2004b), this observation has not been confirmed in larger studies. It is conceivable that the regular use of antioxidant supplements could achieve steady state levels that might interact with chemotherapy or radiation therapy. Nonetheless, the dose, duration, and particular type of antioxidant that might be able to create an interactive effect have not yet been established.
The bioavailability of antioxidant compounds varies according to their source, route of administration, and form (Ratnam, et al., 2006). Lycopene, for example, is more readily absorbed in cooked rather than raw form, and intravenous administration of vitamin C has a biological activity that differs from its oral form (Padayatty, et al., 2004; Ratnam, et al., 2006).
Studies evaluating antioxidant supplementation during chemotherapy are further complicated by the individual variation in the genes that code for antioxidant enzymes as well as variation in the enzymes involved in the metabolism of chemotherapeutic agents, thus potentially impacting the effectiveness of the antioxidant. Individuals with limited or no activity in specific antioxidant genes, such as glutathione-S-transferase, may have decreased ability to exert antiradical activity which may impact the incidence of toxicity and influence treatment outcomes (Ambrosone, et al., 2005; Davies, et al., 2001). The overall antioxidant status of the patient will further impact their effectiveness. Children with acute lymphoblastic leukemia with a higher antioxidant status, measured by the oxygen radical absorbance assay, have been shown to experience fewer side effects associated with cancer therapy (Kennedy, et al., 2004b). The safety of antioxidant supplementation during chemotherapy and radiation therapy is likely to depend on the specificity of the antioxidant and the chemotherapy agent. The opinion that all antioxidants are contraindicated within the context of anticancer treatment is narrow and oversimplified.
Although there is extensive preclinical data supporting a possible role for antioxidant supplements as anticancer agents, the evidence from clinical trials remains quite limited. In a systematic review of studies that evaluated the effects of antioxidants during chemotherapy, six studies investigated the effects of antioxidant supplementation on recurrence rates and survival (Ladas, et al., 2004). Two studies reported survival benefits with antioxidant supplementation (Jaakkola, et al., 1992; Lockwood, Moesgaard, Hanioka, & Folkers, 1994), while one study found antioxidant supplementation was associated with short-, but not long-term survival advantage (Lamm, et al., 1994). In three studies, no overall survival benefit was observed with antioxidant supplementation (Clemens, et al., 1997; Legha, et al., 1982; Mills, 1988). Several clinical studies that have evaluated antioxidants as either primary or adjunctive cancer treatment are presented in the following paragraphs.
Vitamin C, a strong reducing agent, functions as a metal chelator and cellular protector. Vitamin C was postulated to have an important role in the treatment of cancer, based upon the observations that vitamin C is involved in host resistance to cancer and that patients with cancer are often found to be depleted of vitamin C. Supplementation with vitamin C was suggested for its potential role to increase host resistance to cancer by stimulating immune function, increasing resistance to intercellular ground substance hydrolysis by hyalurinidase elaborated by tumor cells, stabilizing the production of hormones, and by protecting the pituitary–adrenal axis from the effects of stress (Cameron, Pauling, & Leibovitz, 1979; Wittes, 1985). Case studies of terminal-stage cancer patients treated with a combination of high-dose oral and intravenous vitamin C who demonstrated prolonged survival were reported (Cameron & Pauling, 1976; Cameron & Pauling, 1978; Cameron, et al., 1979; Cameron, 1991). Two subsequent double-blind randomized placebo- controlled trials showed no survival advantage with high-dose oral vitamin C supplementation as a treatment for cancer (Creagan, et al., 1979; Moertel, et al., 1985). More recent work has shown that vitamin C is cytotoxic to tumor cell lines at concentrations that can be achieved in plasma only by intravenous administration (Koh, et al., 1998). Intravenous vitamin C is also associated with better tissue distribution and saturation (Padayatty, et al., 2004). Whether similar effects would occur in vivo has not been addressed. Recent case studies have reported a beneficial effect of intravenous administration of vitamin C on tumor control (Padayatty, et al., 2006). Clinical studies with intravenous vitamin C are currently in progress.
Vitamin C has also been investigated as an adjunctive agent to conventional chemotherapy. The combination of melphalan, arsenic trioxide, and intravenous vitamin C was shown to be feasible and associated with considerable objective responses in a phase II study in relapsed or refractory multiple myeloma (Berenson, et al., 2006).
Melatonin is an endogenous hormone that is synthesized and secreted by the pineal gland from the amino acid tryptophan. Melatonin protects against oxidative stress through its ability to upregulate antioxidant enzymes such as superoxide dismutases, peroxidases, and enzymes of glutathione supply; to downregulate prooxidant enzymes such as nitric oxide synthases and lipoxygenases; and also to govern some of the actions of quinone reductase 2 (Hardeland, 2005). In addition to its antioxidant effects, melatonin stimulates apoptosis, reduces tumor growth factors, decreases endothelial growth factor, and exerts anti-inflammatory properties (Lissoni, 2002). At physiologically attainable concentrations, melatonin inhibits cancer cell division and with administration of higher oral doses (20–40 mg/day), melatonin is cytotoxic (Mahmoud, Sarhill, & Mazurczak, 2005).
A systematic review has suggested that melatonin supplementation may improve survival in a number of solid tumors (Mills, 2005). Although the effects of melatonin on prolonging survival are intriguing, the studies demonstrating cancer survival were all conducted by the same research group and thus should be confirmed in larger phase III trials.
Melatonin has also been studied as an adjunctive agent to chemotherapy. The addition of melatonin to interferon therapy in the treatment of 22 patients with progressive metastatic renal cell carcinoma was associated with remission in seven patients (three complete) and achievement of stable disease in nine others (Neri, et al., 1994). In conjunction with cisplatin and etoposide, melatonin has also been studied as a treatment for nonsmall cell lung cancer (Lissoni, Chilelli, Villa, Cerizza, & Tancini, 2003). The addition of melatonin to irinotecan in 30 patients with metastatic colorectal cancer who were progressing on 5-fluorouracil therapy was well-tolerated and was associated with partial responses to treatment and the ability to decrease irinotecan dose by 50% without attenuating its efficacy (Cerea, et al., 2003).
The administration of antioxidant mixtures has been investigated in the treatment of several different cancer types, with largely insignificant results.
• A randomized trial of chemotherapy alone or chemotherapy with antioxidants [vitamin C 6100 mg/day; dl-α-tocopherol 1050 mg/day; and β-carotene 60 mg/day] for treatment of 136 patients with stage IIIb and stage IV non–small cell lung cancer observed no difference in tumor response rate or chemotherapy associated toxicities (Pathak, et al., 2005).
• A case–control study investigating megadoses of vitamins in 90 women with unilateral nonmetastatic breast cancer noted no improvement in disease-free survival in the supplemented group; in fact, the supplemented group had lower survival overall, albeit nonsignificantly (Lesperance, et al., 2002).
• In men with androgen-insensitive prostate cancer, combination antioxidant treatment (750 mg of vitamin C, 200 mg selenium, 350 mg vitamin E, and 200 mg of coenzyme Q10) was not associated with significant effects on PSA levels (Hoenjet, et al., 2005).
• However, building on reports of prolonged remission in two women with advanced epithelial ovarian cancer supplementation with vitamin C (3000–9000 mg/day), vitamin E (1200 IU/day), β-carotene (25 mg/day), and vitamin A (5000–10,000 IU/day) (Drisko, et al,. 2003b), a randomized, controlled trial is currently underway (Drisko, et al., 2003a).
Several trials have investigated the efficacy of antioxidants as supportive care agents in individuals receiving conventional anticancer therapy. While many trials investigating the role of antioxidants as supportive care agents have been published, very few have been double-blind randomized trials (Tables 9.2 and 9.3). Summarized here are the results from select studies of antioxidants for cancer-related toxicities, categorized by symptoms.
Cancer-related wasting, or cachexia, is characterized by early satiety, weight loss, anemia, and asthenia (Langer, Hoffman, & Ottery, 2001). A variety of tumor-related factors and increased catabolism often incite cachexia, and its progression is associated with the depletion of intracellular glutathione (GSH) as well as increases in markers of oxidative stress. Supplementation with a GSH-repleting agent has been shown to be effective in treating cachexia associated with human immunodeficiency virus infection (Pacheo, Goldhart, Guilford, Kwyer, & Kongshavn, 1997). A small pilot study in children with cancer at high risk for developing cachexia investigated the effects of an undenatured whey-protein derivative that provides a form of GSH precursor that can be efficiently utilized by cells. Improvements in clinical status including weight gain and increased levels of reduced glutathione were observed (Melnick, et al., 2005).
Supplementation with a mixture of antioxidants may also prevent or ameliorate cancer cachexia. A phase II open label study that investigated the efficacy and safety of a multiagent protocol that included the antioxidants α-lipoic acid (300 mg), vitamin E (400 mg), and vitamin C (500 mg) in 44 adult patients with various malignancies was associated with significant increases in appetite, body weight, and lean body mass (Mantovani, et al., 2006).
Table 9.2. Studies of Antioxidant Supplements for Supportive Care during Cancer Therapy in Adults.
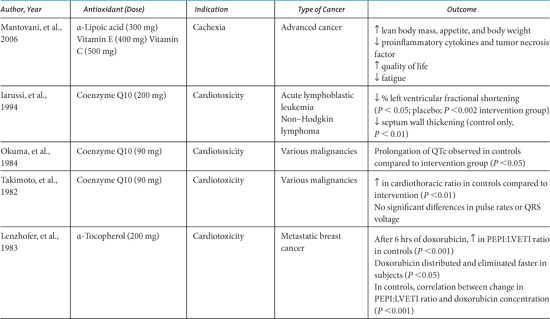
Table 9.3. Studies of Antioxidant Supplements for Supportive Care during Cancer Therapy in Children.
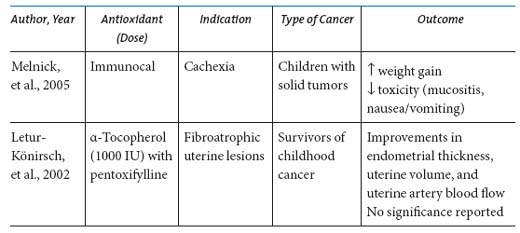
Cardiomyopathy with ventricular failure is a significant cause of long-term morbidity in patients treated with anthracycline chemotherapeutic agents. Coenzyme Q10 (CoQ10), also known as ubiquinone, is an endogenous compound that functions as an antioxidant, promotes membrane stabilization, and acts as a cofactor in many metabolic pathways, including the production of adenosine triphosphate in oxidative respiration. The most researched antioxidant supplement for the prevention of anthracycline-induced cardiotoxicity, CoQ10, may have a cardioprotectant effect by replenishing or scavenging free radicals in cardiac myocytes (Greenberg & Frishman, 1990). Plasma CoQ10 levels have been observed to be low in patients with melanoma, breast, and lung cancer (Folkers, Osterborg, Nylander, Morita, & Mellstedt, 1997; Rusciani, et al., 2006; Shinkai, et al., 1984). A systematic review of clinical trials investigating the efficacy of CoQ10 reported some benefits; however, many of the trials were hampered by small sample sizes, inclusion of patients with a mixture of cancer diagnoses, and poor study design or methodology (Roffe, Schmidt, & Ernst, 2004).
Four separate clinical studies have evaluated vitamin E supplementation for cardioprotection. Although one study reported improvements in cardiac function with nifedipine and vitamin E supplementation (α-tocopherol 200 mg by intramuscular route) (Lenzhofer, Ganzinger, Rameis, & Moser, 1983) given to women with metastatic breast cancer, cardioprotective effects of vitamin E were not confirmed in three other studies (Legha, et al., 1982; Wagdi, Fluri, Aeschbacher, Fikrle, & Meier, 1996; Weitzman, Lorell, Carey, Kaufman, & Stossel, 1980).
Neurotoxicity is a frequent chemotherapy dose-limiting toxicity in patients treated with vinca alkaloids (vincristine, vinblastine) or platinum complexes, particularly cisplatin. Decreased vitamin E levels have been observed in patients who have received treatment with cisplatin (Weijl, et al., 1998) and vitamin E deficiency has clinical similarity to cisplatin-induced neuropathy (Leonetti, et al., 2003). Animal studies in male CD-1 nude mice reported that vitamin E decreased cisplatin-induced neuropathy by neutralizing oxidative stress that occurs at the dorsal root ganglia, the target of cisplatin neurotoxicity, as measured by histologic analysis (Leonetti, et al., 2003). In a small pilot study, neurotoxicity developed in only 4 of 16 patients supplemented with vitamin E along with cisplatin, paclitaxel, or their combination regimens, versus 11 of 15 patients receiving chemotherapy alone (P = 0.019, overall RR= 0.34) (Argyriou, et al., 2005). In a randomized controlled trial, vitamin E supplementation was associated with a reduction in the incidence of paclitaxel-induced neuropathy (Argyriou, et al., 2006).
Otoxicity, a dose-limiting toxicity of cisplatin therapy, can develop with oxidative stress-induced injury in the organ of Corti. (Ravi, Somani, & Rybak, 1995) Although reductions in plasma antioxidant status in patients receiving cisplatin-based chemotherapy have been observed (Weijl, et al., 1998), only one clinical trial has been completed to investigate the effects of antioxidant supplementation in preventing hearing loss. In a study of 48 patients treated with cisplatin-based therapy and a mixture of antioxidants (1000 mg vitamin C, 400 mg dl-α-tocopherol acetate, and 100 µg selenium), no significant differences in the incidence of ototoxicity, nephrotoxicity, or bone marrow toxicity were observed, despite the observation of elevated levels of plasma antioxidants in the intervention group (Weijl, et al. 2004). A significant correlation, unrelated to the treatment group, was observed between higher reduced–oxidized vitamin C ratios and lower malondialdehyde levels—markers of oxidative stress—and the incidence of otoxicity and nephrotoxicity. This study was complicated by the poor compliance to the treatment assignment, as 64% and 46% of subjects, respectively, did not adhere to antioxidant protocol in the intervention and placebo arms.
Reductions in plasma levels of antioxidants in patients undergoing treatment for cancer or receiving conditioning regimens in preparation for stem cell transplants have been observed (Ladas, et al., 2004), leading researchers to investigate the effect of different mixtures of antioxidants on frequently encountered chemotherapy-related toxicities, such as anemia and myelosuppression. In a study of women being treated for ovarian cancer, supplementation with selenium (50 µg), vitamin C (200 mg), vitamin E (36 mg), and β-carotene (15 mg) was associated with increases in neutrophil counts and decreased incidence of chemotherapy-associated side effects (Sieja, 2000). While one study reported improvements in blood indices in patients supplemented with 4000 µg of selenium, (Hu, et al., 1997) another noted no difference in the incidence of general toxicities in a group of patients who took supplements containing d-α-tocopherol (3200 IU) (Blanke, et al., 2001). Significant improvements in neutrophil recovery was observed among 35 women with breast cancer who took either a multivitamin or vitamin E, although decreased neutrophil recovery was observed in the women in this study taking folic acid (Branda, et al., 2004). Additionally, supplementing with vitamin C (500 mg) and vitamin E (400 mg) in an attempt to prevent tamoxifen-induced hypertriglyceridemia was associated with improvements in plasma lipid and lipoprotein levels in postmenopausal women with resectable breast cancer (Babu, et al., 2000).
Because one of the major ways that radiation therapy exerts its anticancer effect is by generating free radicals, there has been much controversy and uncertainty surrounding the use of antioxidants during radiation treatment. To date, several research studies have investigated the effect of different antioxidant mixtures on the risk of developing acute and long-term radiation-associated side effects and their ability to influence survival.
A few small trials have investigated the effect of antioxidants for the prevention of proctitis or enteritis, tissue induration, and mucositis associated with radiation therapy.
• Twenty-one patients with grade I–II radiation-induced proctitis/enteritis were treated with a combination of vitamin E and pentoxifyl-line, a radiosensitizing agent (Hille, et al., 2005). In the pentoxifylline/vitamin E treatment group, 15 of 21 patients (71%) experienced a reduction of symptoms, with 7 patients achieving a reduction from grade I/II to grade o toxicity and 8 achieving a reduction from grade II to grade I toxicity.
• Grape seed proanthocyanidin extract was administered for 6 months to 66 women for the prevention of tissue induration following radiation to the breast (Brooker, et al., 2006). No significant differences were observed at 6 and 12 months following completion of radiation.
• Other studies have observed that the topical application of β-carotene (250 mg) or topical vitamin E oil (400 mg) for the prevention of mucositis is associated with reductions in the severity or duration of mucositis (Mills, 1988; Wadleigh, et al., 1992).
• A small case series investigating the effect of antioxidants among survivors of childhood cancer reported that six women who had received pelvic radiation as children had reductions in fibroatrophic uterine lesions following the administration of vitamin E with pentoxifylline.
A survival benefit was reported with pentoxifylline and vitamin E supplementation in a randomized nonplacebo controlled trial among 66 patients receiving radiotherapy for stage IIIB non–small cell lung cancer (Misirlioglu, Erkal, Elgin, Ugur, & Altundag, 2006). In the group receiving the supplements, 2-year overall survival and progression-free survival were 30% and 23%, respectively, versus 18% and 14%, respectively for the control group (P = 0.0175; P = 0.0223, respectively).
In a double-blind randomized controlled trial among 54 patients with head and neck cancer, an orally administered vitamin E rinse during radiotherapy was associated with a 36% risk reduction in symptomatic mucositis. (Ferreira, et al., 2004) However, 2-year overall survival was reduced in the supplementation group (vitamin E group: 32.2%; placebo group 62.9%), although this difference was not statistically significant.
A single large, well-designed trial investigated the efficacy of antioxidant supplementation for prevention of radiotherapy associated mucositis and reduction of second primary cancers in 540 head and neck cancer patients during radiation therapy (Bairati, et al., 2005). Patients took the antioxidant supplements, dl-α-tocopherol (400 IU/day) and β-carotene (30 mg/day), both during and for 3 years following the completion of radiation therapy. β-Carotene supplementation was discontinued early due to ethical concerns following the observations of significantly increased risks of lung cancer in patients supplemented with antioxidants that included β-carotene in large cancer chemoprevention trials (The Alpha-Tocopherol, Beta-carotene Cancer Prevention Study Group, 1994; Omenn, et al., 1996). In this study, patients in the intervention arm experienced less severe acute side effects from radiation therapy. The rate of local recurrence was higher among patients randomized to antioxidant supplementation, although the increased risk was seen in patients receiving the combination of dl-α-tocopheral and β-carotene, rather than those patients assigned to dl-α-tocopheral alone. With longer follow-up, overall survival was significantly compromised in the antioxidant supplemented group. Further analyses demonstrated that all-cause mortality was significantly increased in the supplement arm (hazard ratio: 1.38, 95% confidence interval 1.03–1.85), and cause-specific mortality rates tended to be higher in the supplement arm than in the placebo arm (Bairati, et al., 2006). Other investigators have countered that differences in doses, sources (synthetic vs. natural antioxidants), and dose schedules may have accounted for the adverse effects of antioxidant supplementation on long-term prognosis in this trial (Prasad & Cole, 2006).
Clinical studies of antioxidant supplementation and changes in oxidative status, disease risk, or disease outcome have been performed among healthy individuals, populations at risk for cancer, and patients undergoing cancer treatment. However, when the evidence is evaluated as a whole, the published clinical trials have not incorporated consistent sample populations, utilized standardized treatment regimens, or reported consistent outcomes. These inconsistencies preclude definitive conclusions in regard to the safety and effi-cacy of antioxidant supplementation for chemotherapy- or radiation therapy-related toxicities or survival from cancer. Until crucial additional clinical trials are completed, algorithms for evaluating possible interactions of supplements and conventional cancer therapies, as well as systematic approaches to review the published literature may serve as guides (Seely, Stempak, & Baruchel, 2007; Weiger, et al., 2002). Broad rejection or recommendation for the concurrent use of antioxidants with chemotherapy or radiation therapy is not justified at the present time.
Recommendations for clinical practice at the current time include the following:
• Since the clinical research on antioxidant supplementation has not yet adequately demonstrated that the benefits of supplementation clearly outweigh the risks, the possibility of harm must be strongly considered.
• Patients should therefore be advised to avoid dietary antioxidant supplements above the basic nutritional requirements during radiation therapy.
• Patients should exert caution in supplementing with antioxidants while receiving treatment with chemotherapy agents (Table 9.1) until their combined use is found to be safe and does not compromise the efficacy of chemotherapy agents.
There are theoretical reasons to support that the role for some antioxidants in either the enhancement of the effects of some chemotherapy or radiation regimens or the reduction of treatment-related toxicities, without interfering with anticancer activity. Future trials evaluating the safety and efficacy of antioxidants must consider the diagnosis, the specific type of conventional treatment, and the type and form of antioxidant to be used.
Suggestions for future antioxidant research include the following:
• Preliminary evidence supports a beneficial role for melatonin during cancer treatment; large randomized phase III trials cohorts should be performed to confirm.
• Despite the widespread prevalence of cachexia in patients with cancer, no consistent approaches have been developed. Future research should investigate potential antioxidant treatments and identify the appropriate mixtures, dosing, duration, and timing of supplementation.
• Findings from a meta-analysis support the efficacy of coenzyme Q10 for its cardioprotectant effects; this finding needs to be investigated in a large, phase III randomized trial with appropriate markers of acute and long-term cardiotoxicity.
• Antioxidants may adversely affect outcome in patients with head and neck cancer receiving radiation therapy. Additional trials are needed to ascertain the safety of antioxidant supplementation in patients with other types of malignancies undergoing radiation treatment.
• Antioxidant status may impact risk for developing chemotherapy-related toxicities, particularly in patients treated with antioxidant-depleting regimens. Further research is needed to evaluate the role of antioxidant supplementation for supportive care, which may allow administration of the recommended doses of conventional therapies and minimization of therapy delays and dose reductions. However, before broad recommendations can be made for antioxidant supplementation for the prevention or treatment of general chemotherapy-related toxicities, more research is needed to determine the biological mechanisms, appropriate antioxidant mixtures and dosing for each clinical setting.
• The majority of published clinical trials have been performed in adults with cancer. Investigations of antioxidant supplementation in children and adolescents with cancer are also needed.
Bairati I, Meyer F, Jobin E, Gelinas M, Fortin A, Nabid A, et al. (2006). Antioxidant vitamins supplementation and mortality: A randomized trial in head and neck cancer patients. International Journal of Cancer, 119, 2221–2224.
Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, & Gyllenhaal C. (2007). Impact of antioxidant supplementation on chemotherapeutic efficacy: A systematic review of the evidence from randomized controlled trials. Cancer Treat Reviews, 33(5), 407–418.
Cameron E, Pauling L, & Leibovitz B. (1979). Ascorbic acid and cancer: review. Cancer Research, 39, 663–681.
Ladas EJ, Jacobson JS, Kennedy DD, Teel K, Fleischauer A, & Kelly KM. (2004). Antioxidants and cancer therapy: A systematic review. Journal of Clinical Oncology, 22, 517–528.
Moertel CG, Fleming TR, Creagan ET, Rubin J, O’Connell MJ, & Ames MM. (1985).
High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison. The New England Journal of Medicine, 312, 137–141.
Moss RW. (2006). Should patients undergoing chemotherapy and radiotherapy be prescribed antioxidants? Integrative Cancer Therapies, 5, 63–82.
Prasad KN & Cole WC. (2006). Antioxidants in cancer therapy. Journal of Clinical Oncology, 20;24, e8–e9.
Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, & Kumar MN. (2006). Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. Journal of Controlled Release, 20;113, 189–207.
Rock CL, Newman VA, Neuhouser ML, Major J, & Barnett MJ. (2004). Antioxidant supplement use in cancer survivors and the general population. The Journal of Nutrition, 134, 3194S–3195S.
Seely D, Stempak D, & Baruchel S. (2007). A Strategy for Controlling Potential Interactions Between Natural Health Products and Chemotherapy: A Review in Pediatric Oncology. Journal of Pediatric Hematology and Oncology, 29, 32–47.
(A complete reference list for this chapter is available online at http://www.oup.com/us/integrativemedicine).