10
Physical Activity and Cancer
CLARE STEVINSON AND KERRY S. COURNEYA
KEY CONCEPTS
 Research has established that physical activity plays an important role in the prevention and management of many chronic medical conditions.
Research has established that physical activity plays an important role in the prevention and management of many chronic medical conditions.
 Physical activity helps to protect against colon and breast cancer, and possibly endometrial, lung, and prostate cancer.
Physical activity helps to protect against colon and breast cancer, and possibly endometrial, lung, and prostate cancer.
 Physical activity is essential for maintaining a healthy body weight, which is important since obesity is a major risk factor for many cancers.
Physical activity is essential for maintaining a healthy body weight, which is important since obesity is a major risk factor for many cancers.
 Some preliminary data suggest that postdiagnosis physical activity may be associated with increased survival in colon and breast cancer.
Some preliminary data suggest that postdiagnosis physical activity may be associated with increased survival in colon and breast cancer.
 Physical activity can improve general health and strength, contributing to enhanced functional status and ability to cope with disease- and treatment-related symptoms and side effects.
Physical activity can improve general health and strength, contributing to enhanced functional status and ability to cope with disease- and treatment-related symptoms and side effects.
 Current evidence suggests that by adhering to a graded exercise program, cancer survivors can maintain or increase their level of conditioning function and thereby avoid becoming trapped in a perpetuating cycle of deteriorating physical function and increasing fatigue.
Current evidence suggests that by adhering to a graded exercise program, cancer survivors can maintain or increase their level of conditioning function and thereby avoid becoming trapped in a perpetuating cycle of deteriorating physical function and increasing fatigue.
 Physical activity can also enhance quality of life, and help to prevent other chronic conditions (e.g., diabetes or cardiovascular disease) for which cancer survivors are at increased risk.
Physical activity can also enhance quality of life, and help to prevent other chronic conditions (e.g., diabetes or cardiovascular disease) for which cancer survivors are at increased risk.
 The risk/benefit profile of physical activity is favorable, assuming that key safety issues are considered for each individual before initiating an exercise program.
The risk/benefit profile of physical activity is favorable, assuming that key safety issues are considered for each individual before initiating an exercise program.

A growing body of research has established an important role for physical activity in the prevention and management of various chronic medical conditions, including coronary heart disease (Taylor, et al., 2004), stroke (Gordon, et al., 2004), hypertension (Pescatello, et al., 2004), noninsulin dependent diabetes (Albright, et al., 2000), obesity (Jakicic, et al., 2001), musculoskeletal disorders (Vuori, 2001), and mental health problems (Callaghan, 2004). Evidence of the effects of physical activity in protecting against cancer has been accumulating over the last five decades. More recently, attention has also turned to the potential benefits of exercise for individuals already diagnosed with cancer.
In 2001, Courneya and Friedenreich published the PEACE (Physical Exercise Across the Cancer Experience) framework (Fig. 10.1), identifying key periods during which exercise has potential benefit (Courneya & Friedenreich 2001). These include the primary prevention of cancer, preparing for cancer treatments, coping with side effects during treatment, rehabilitation after treatment, long-term health promotion for those with good outcomes, and palliative care for those unlikely to be cured. The majority of research so far has focused on three broad questions: (1) Can physical activity help prevent cancer? (2) Can physical activity contribute to increased survival in those diagnosed with cancer? (3) Can physical activity improve quality of life outcomes in patients during and after cancer therapy? This chapter summarizes the evidence available so far in these areas and provides guidelines for appropriate levels of physical activity for cancer prevention and rehabilitation.
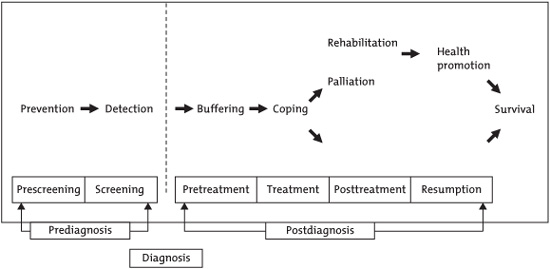
FIGURE 10.1. Framework PEACE: An organizational model for examining physical exercise across the cancer experience. Reprinted with permission from Courneya KS & Friedenreich CM. (2001). Framework PEACE: An organizational model for examining physical exercise across the cancer experience. Annals of Behavioral Medicine, 23(4), 263–272. Copyright 2001, Lawrence Erlbaum Associates, INC.
Cancer Prevention
The evidence for a preventative role of physical activity against cancer has been accumulating since a seminal study of railway workers in 1962 that demonstrated a 30% reduced risk in cancer mortality among section men, compared with clerks (Taylor, et al., 1962). A comprehensive review published by the American Institute for Cancer Research in 2005 (AICR, 2005) concluded that the totality of evidence from more than 180 epidemiological studies confirms a protective effect. Furthermore, it reported no consistent evidence of an increased risk of any cancer in association with physical activity. In terms of specific cancers, the protective effect was classified as “convincing” for colon and breast cancer. The results of a meta-analysis of 47 studies indicate a risk reduction for colon cancer of approximately 20% but no effect on rectal cancer (Samad, Taylor, Marshall, & Chapman, 2005). The AICR concluded that the evidence for a protective role of physical activity was “probable” for endometrial cancer and “possible” for prostate and lung cancer. Further evidence of a preventive effect for lung cancer is provided by a meta-analysis of nine studies indicating a reduced risk with higher levels of leisure-time physical activity (Tardon, et al., 2005). However, a recently updated meta-analysis of 41 prostate cancer studies found the evidence still weak and inconsistent (Lee, 2006). Although reduced risk in association with physical activity has been observed for other cancers (e.g., testicular, ovarian, kidney, pancreatic), the AICR report considered the evidence to be “insufficient” because of the small number of studies.
MECHANISMS
The reasons for the apparent cancer-protective effects of physical activity have not yet been determined. It is conceivable that independent factors that influence both ability to exercise, and susceptibility to cancer, might explain the relationship, such as preexisting disease or healthy lifestyle. However, there are also various plausible biological explanations, and research efforts are focused on understanding the role of these potential mechanisms (Rundle, 2005). Table 10.1 summarizes the possible mechanisms operating at either a systemic level to reduce overall cancer risk or a site-specific level to help prevent particular cancers. The role of physical activity in maintaining a healthy body weight is considered particularly important since obesity is a major risk factor for many cancers. However, results of studies that have controlled for energy intake or body size indicate that activity influences cancer risk independently of weight control. It is considered most likely that multiple mechanisms are responsible for the anticarcinogenic properties of physical activity (Westerlind, 2003).
Table 10.1. Potential Biological Mechanisms for Protective Effect of Physical Activity against Cancer.

The American Institute for Cancer Research reports that obesity increases the risk for breast, colon, endometrial, esophageal, kidney, and prostate cancers by 25 to 33 percent.
Cancer Survival
Given the important role identified for physical activity in the primary prevention of cancer, it is conceivable that the mechanisms thought to be responsible for this, may also play a part in secondary prevention. However, there is, so far, only preliminary evidence to indicate that exercise may directly influence disease progression or survival (Courneya, et al., 2004).
Results from animal studies are ambiguous in this respect. Although several studies demonstrate that exercise protects against tumor metastasis in rodents (Hoffman, 1994), findings are strongly influenced by the specific tumor and animal models used (Hoffman, 2003). Furthermore, the high volume of the exercise protocols used in many of these studies does not represent a realistic level of physical activity for human cancer patients, hence the generalizability of the findings is severely limited.
A small number of clinical trials with cancer patients have examined the effect of exercise interventions on intermediate biological markers that are thought to lie on the causal pathway between physical activity and cancer recurrence or mortality. There is some evidence of increases in natural killer cell cytotoxic activity and other immune function parameters following exercise programs, beyond that expected through normal recovery (Fairey, Courneya, Field, & Mackey, 2002; Fairey, et al., 2005). Changes in sex steroid hormones such as estrodial, estrone, and testosterone were not observed in studies of breast and prostate cancer patients (McTiernan, et al., 1998; Segal, et al., 2003), which may reflect the effects of hormonal treatments received by these patients. In two trials examining the effects on metabolic hormones, fasting glucose, insulin, and insulin resistance were not influenced by aerobic (Fairey, et al., 2003) and resistance (Schmitz, Ahmed, Hannan, & Yee, 2005) exercise programs in breast cancer survivors. Although, few studies exist so far, this is an area generating considerable interest, leading to a growth in transitional research that attempts to validate biomarkers, and understand the effects of exercise on these intermediate outcomes, in order to determine the clinical implications for patients in terms of disease progression and survival.
The most encouraging data suggesting that physical activity may improve cancer survival, come from epidemiological investigations. In one of the first studies to directly address this question, 2987 women who were diagnosed with breast cancer while participating in the Nurses Health Study, were monitored for up to 18 years, with physical activity assessed every 2 years (Holmes, et al., 2005). After adjusting for various demographic and medical variables, women who reported 9 to 15 metabolic equivalent task (MET)-hours of physical activity per week (equivalent to 3–5 hours of brisk walking), had a 50% decreased risk of cancer-specific mortality, compared with women who reported less than 3 MET-hours per week (e.g., 1 hour of walking). Similar risk reductions were observed for breast cancer recurrence and all-cause mortality.
Another analysis from the Nurses Health Study involved 573 women diagnosed with colorectal cancer (Meyerhardt, et al., 2006). Disease-specific mortality and all-cause mortality were reduced by approximately 50% in women performing at least 18 MET-hours per week of physical activity, compared with those reporting less than 3 MET-hours per week. The analyses indicated that it was postdiagnosis physical activity that was related to survival, and not activity performed before disease onset.
In a further study, 816 patients with stage III colon cancer who were part of a clinical trial of adjuvant chemotherapy completed physical activity measures during treatment and 6 months afterward (Meyerhardt, et al., 2006). Increases in recurrence-free survival, disease-free survival, and overall survival were observed in association with increasing volumes of physical activity. After controlling for various demographic and medical variables, those performing at least 18 MET-hours of exercise per week had a 49% reduction in risk of recurrence or death compared with those performing less than 3 MET-hours per week, over a 3-year period following treatment.
The implication from these studies that a lifestyle factor such as physical activity can have prognostic value is of obvious interest and importance. Results from further studies investigating the impact of physical activity performed after diagnosis on disease progression and survival are keenly awaited.
Cancer Rehabilitation
Regardless of whether or not physical activity will ultimately prove to have specific effects on disease or survival outcomes, there is, nonetheless, increasing acceptance of the importance of cancer patients being physically active in order to maximize their functional status and cope with disease- and treatment-related symptoms.
Cancer treatments such as surgery, chemotherapy, and radiation, typically involve damage to healthy tissue as well as the destruction of malignant cells. Patients may experience a range of possible side effects (e.g. pain, nausea, fatigue, alopecia, neutropenia, peripheral neuropathy), and psychological sequelae (e.g. anxiety, depression). Furthermore, overall physical function is generally diminished due to losses of aerobic capacity, muscle tissue, and range of motion. Therefore, great importance is placed on preserving physical and psychosocial functioning as far as possible during treatment and restoring it afterward.
The results of a recent survey illustrate the reduction in physical function experienced by cancer patients. The physical performance limitations of 279 short-term (<5 years) and 434 long-term (≥5 years) cancer survivors were compared with 9370 individuals with no cancer history (Ness, Wall, Oakes, Robison, & Gurney, 2006). The results indicated that over half of the cancer survivors (54% of short-term and 53% of long-term survivors) had performance limitations, compared with 21% of the individuals with no cancer history. The most commonly reported difficulties were crouching/kneeling, standing for 2 hours, lifting/carrying 10 pounds, and walking quarter of a mile. Given the importance of such actions to performing activities of daily living (e.g., dressing, housework, childcare, shopping, gardening), there is a clear need for rehabilitation interventions focusing on building physical endurance and strength. Furthermore, this study demonstrates that these needs are not restricted only to patients who have recently completed treatment but also apply to longer-term survivors.
EVIDENCE BASE
Since the pioneering work of Maryl Winningham of Ohio State University College of Nursing during the 1980s (Winningham, 1983; Winningham, MacVicar, & Burke, 1986) the number of exercise intervention studies with cancer patients has grown exponentially. There are now at least 20 review articles published that attempt to summarize the results and implications of individual studies. Table 10.2 lists the conclusions of systematic reviews published since 2004 that have evaluated the evidence from intervention trials (Oldervoll, Kaasa, Hjermstad, Lund, & Loge, 2004; Stevinson, Lawlor, & Fox, 2004; Douglas, 2005; Galväo & Newton, 2005; Knols, Aaronson, Uebelhart, Fransen, & Aufdemkampe, 2005; Schmitz, et al., 2005; Conn, Hafdahl, Porock, McDaniel, & Nielsen, 2006; McNeely, et al., 2006a).
Conclusions from reviews have been consistently positive regarding the potential benefits of physical activity during cancer therapy and after treatment completion. Nonetheless, all include caveats regarding the inconsistencies among results and the heterogeneity and methodological limitations of trials. Encouragingly, methodological rigor has improved in more recent trials, demonstrating more modest outcomes, but increasing the validity of the reported findings.
PHYSICAL FUNCTION
The most consistent and positive effects demonstrated in randomized clinical trials relate to physical function outcomes. Results of meta-analyses show moderate improvements in cardiorespiratory fitness among cancer survivors engaging in aerobic exercise programs during, and after, cancer treatment (Stevinson, et al., 2004; Schmitz, et al., 2005; Conn, et al., 2006; McNeely, et al., 2006a). The benefits of preserving fitness during treatment, and gradually increasing it again afterward, are considerable in terms of being able to perform daily activities and continue with leisure pursuits.
Table 10.2. Recent Systematic Reviews of Exercise Interventions for Cancer Survivors.
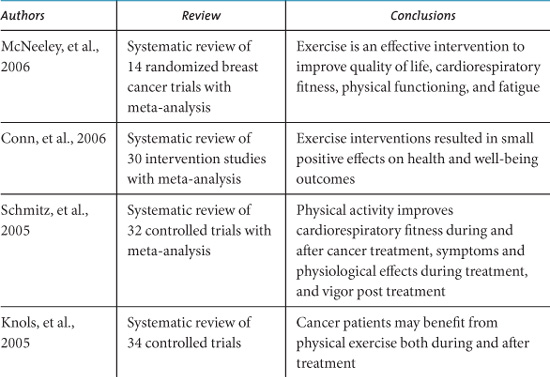
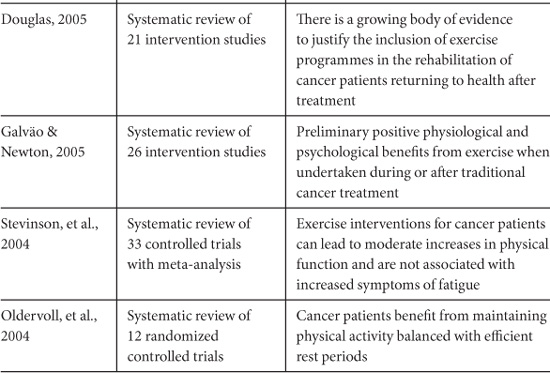
Similarly, encouraging functional outcomes have been demonstrated in trials that have focused on resistance exercise training. Reductions in shoulder pain and disability were reported in head and neck cancer survivors who had undergone spinal accessory neuropraxia/neurectomy (McNeely, et al., 2004), and increases in muscular fitness (Segal, et al., 2003) and muscle mass (Marcora, Oliver, Callow, Lemmy, & Stuart, 2005) were demonstrated for prostate cancer patients receiving androgen deprivation therapy. Increased muscle mass was also reported in breast cancer survivors following a 6-month weight training program (Schmitz, et al., 2005). If resistance exercise helps prevent or reverse the loss of muscle tissue that can result from inactivity, inadequate nutrition, or cachectic processes, it would make a significant contribution to maintaining functional ability.
TREATMENT SIDE EFFECTS
There is some preliminary evidence from meta-analysis that exercise can help in the management of treatment-related symptoms or side effects (Schmitz, et al., 2005). One trial involving inpatients receiving high-dose chemotherapy following peripheral blood stem cell transplantations demonstrated a range of positive outcomes for those exercising daily on a supine cycle ergometer, compared with control participants (Dimeo, Fetscher, Lange, Mertelsmann, & Keul, 1997). These outcomes included lower pain severity and less use of analgesics, lower severity of diarrhea, shorter duration of thrombopenia and neutropenia, and shorter hospitalization.
Cancer-related fatigue has been identified as one of the most common and distressing symptoms reported by patients and also as one of the most difficult to treat (Curt, 2001). An overemphasis on rest carries the risk of increased fatigue, due to individuals becoming caught in a vicious cycle of inactivity leading to further deconditioning, hence greater fatigue upon even minimal exertion. Results of meta-analyses provide encouraging evidence that fatigue is not increased by exercise (Stevinson, et al., 2004; Schmitz, et al., 2005) and may actually be reduced (Conn, et al., 2006; McNeely, et al., 2006a). This is an important finding with respect to the understandable concerns of patients and care-givers that exercise may cause or exacerbate existing fatigue. Instead, current evidence suggests that by adhering to a graded exercise program, cancer survivors can maintain or increase their level of conditioning and function, thereby avoiding becoming trapped in a perpetuating cycle of deteriorating physical function and increasing fatigue. Only one trial has demonstrated greater reductions in fatigue among the control group than the exercisers (Thorsen, et al., 2005). These participants were cancer patients recruited straight after completion of chemotherapy, and the authors suggested that a period of spontaneous recovery before commencing exercise may be optimal for fatigue reduction.
Exercise has been suggested as useful method of reversing unwanted weight gain in cancer survivors (Schwartz, 2000). Although a few studies have reported improved body composition (i.e. improved lean/fat tissue ratio) (e.g. Courneya, et al., 2003; Schmitz, et al., 2005; Winningham, MacVicar, Bondoc, Anderson, & Minton, 1989), there is no evidence of significant changes in total body weight (Schmitz, et al., 2005; McNeely, et al., 2006a). It may be that most clinical trials of exercise have been of insufficient duration to influence body weight, and that this outcome is better addressed in longer-term health promotion protocols. A recent systematic review of 14 trials in breast cancer survivors concluded that the evidence for improved body composition was sketchy, but encouraging (Ingram, Courneya, & Kingston, 2006).
Specific exercises are often prescribed for side effects related to specific cancer types. For instance, pelvic exercises (commonly known as Kegels) can help restore continence following treatment for prostate cancer.
QUALITY OF LIFE
Many trials have assessed the effect of exercise interventions on dimensions of quality of life. Meta-analysis results indicate small advantages in overall quality of life for cancer survivors who exercise, over those in control groups, with the strongest effects demonstrated for those who are post treatment (Schmitz, et al., 2005; Conn, et al., 2006; McNeely, et al., 2006). Several of the more recent and rigorous trials have demonstrated significant quality of life benefits. These studies have included breast cancer survivors post treatment following resistance training (Ohira, Schmitz, Ahmed, & Yee, 2006) and aerobic exercise (Courneya, et al., 2003) interventions, and men with prostate cancer undergoing androgen deprivation therapy and resistance training (Segal, et al., 2003). A heterogeneous sample of cancer survivors who followed a walking intervention in combination with group psychotherapy improved functional aspects of quality of life more than those receiving only group psychotherapy (Courneya, et al., 2003).
Physical Activity Guidelines
CANCER PREVENTION
Many public health organizations provide general recommendations for physical activity in the context of reducing risk of cancer. Table 10.3 summarizes the recommendations of some major international and national cancer organizations. Most advise a minimum of 30 to 60 minutes of moderate to vigorous intensity activity 5 to 7 days per week. These recommendations are in line with general health promotion guidelines regarding physical activity (US Department of Health and Human Services, 1996; Australian Government Department of Health and Ageing, 1999; UK Department of Health, 2004).
Table 10.3. Physical Activity Recommendations for Cancer Prevention from Public Health Organizations.
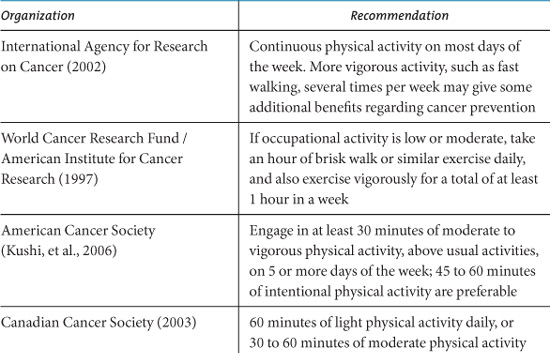
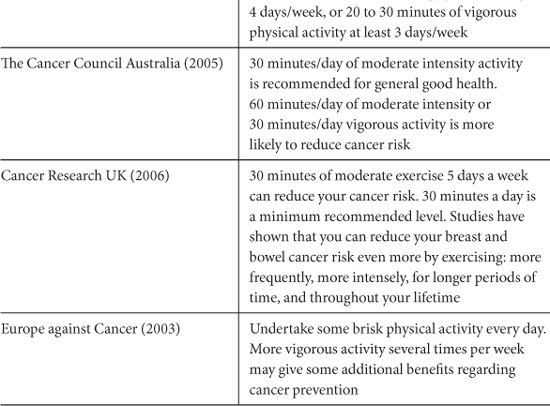
The American Cancer Society recently modified their recommendations to advise a minimum of 30 minutes of moderate to vigorous physical activity, above usual activities, on five or more days of the week, with 45 to 60 minutes of intentional physical activity being preferable (Kushi, et al., 2006). It is likely that public health guidelines will need revision in the future as more precise evidence emerges regarding the optimal frequency, duration, and intensity of physical activity required for risk reduction.
The American Cancer Society makes a general recommendation for adults of at least 30 minutes of moderate physical activity 5 days a week. Further evidence suggests that vigorous physical activity 5 days a week may reduce the risk of breast and colon cancer.
CANCER REHABILITATION
Humpel & Iverson systematically reviewed published guidelines/recommendations regarding exercise for cancer survivors from journal articles, books, and cancer-related websites (Humpel & Iverson 2005). Recommendations for aerobic exercise typically covered a broad range of three to five times weekly for 10 to 60 minutes at a low to moderate intensity. Recommendations for resistance training were within the range of 1 to 3 times weekly with 1 to 3 sets of 8 to 15 repetitions at low resistance. The need to individualize the prescription according to medical treatments, comorbidities, and fitness levels was emphasized in most cases. Although recommendations were generally consistent across the different sources, supportive scientific evidence was not routinely cited and the reviewers concluded that none met criteria for evidence-based guidelines.
In a recent review of the potential therapeutic role of exercise training in the cancer setting, McNeely and colleagues proposed recommendations for integrating exercise programming into clinical practice (McNeely, Peddle, Parliament, & Courneya, 2006b). They suggested formal exercise training as part of a supervised outpatient rehabilitation program immediately following treatment completion. The exercise prescription may involve aerobic training for cardiorespiratory fitness, resistance training for muscular strength and endurance, and flexibility exercises for increasing range of motion and muscle length. An initial program of at least 8 weeks was recommended, with the exercise prescription cautiously progressed over time and allowing for modifications where appropriate. A summary of these guidelines is presented in Figure 10.2.
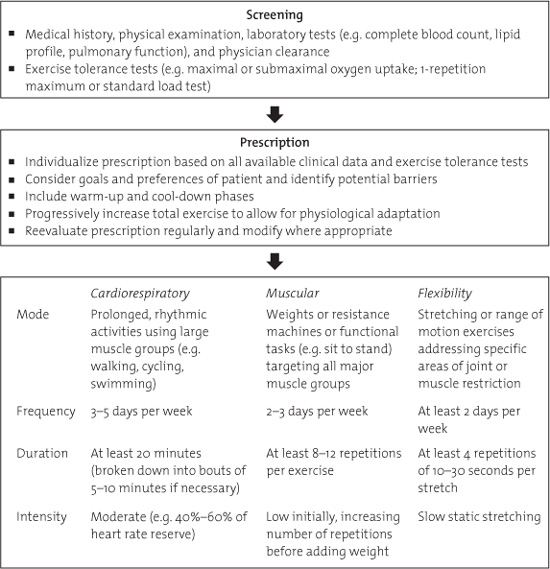
FIGURE 10.2. Recommendations for supervised exercise rehabilitation programming for cancer patients (McNeely, et al., 2006a).
RISKS OF EXERCISE
Clearly, for exercise to be considered a valuable intervention that can be routinely recommended to cancer survivors it must have a positive risk/benefit ratio. The risks associated with exercise at levels required for health promotion are low in the general population. For cancer patients, concerns relate to the possibility of exercise leading to immunosuppression, falls, bone fractures, complications of cardiotoxic treatments, exacerbation of pain and other symptoms, and interference with treatment completion or efficacy. McNeely et al. (2006b) have identified key safety considerations for prescribing exercise to cancer patients, and have provided a list of contraindications and precautions to exercise testing and training (Table 10.4).
Table 10.4. Contraindications and Precautions to Exercise Testing and Training.
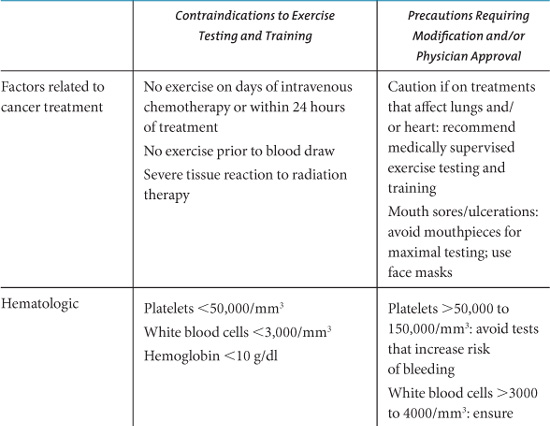


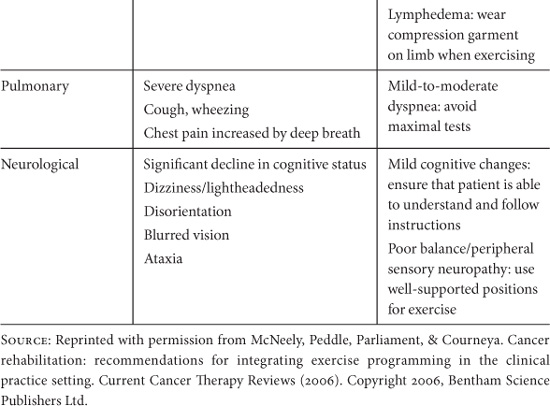
Systematic reviews of trials with cancer survivors have reported few adverse events associated with exercise (Stevinson, et al., 2004; Schmitz, et al., 2005; McNeely, et al., 2006a). However, it should be noted that clinical trials have rigorous screening criteria and generally exclude participants for whom exercise may pose a potential risk (e.g. those with uncontrolled cardiovascular or pulmonary disease, existing musculoskeletal disorders, or cancer-related conditions such as cachexia, anemia, neutropenia, thrombocytopenia, or metastatic bone disease). Studies that have addressed the role of exercise in causing or worsening lymphedema in breast cancer survivors who have undergone axillary node dissection have found no increased risk with upper body physical training (Ahmed, Thomas, Yee, & Schmitz, 2006; Harris & Niesen-Vertommen, 2000; McKenzie & Kalda, 2003).
In assessing the risk/benefit ratio of exercise, it is important to consider the potential harm to cancer survivors of remaining inactive alongside the possible hazards of exercise. Physical inactivity leads to deconditioning, bone loss, and muscle atrophy, decreases in glucose metabolism, insulin sensitivity, digestive function and immunosurveillence, and increases in cardiovascular risk factors (e.g., lipid levels, blood pressure). Maintaining regular activity is essential therefore for reducing the risk of developing other chronic conditions (e.g., diabetes, cardiovascular disease, osteoporosis), and particularly so for cancer survivors who may be at increased risk of further disease (Brown, Brauner, & Minnotte, 1993; Mahon, 1998; Penedo, Schneiderman, Dahn, & Gonzalez, 2004).
Summary
In terms of prevention, there is compelling evidence that regular physical activity reduces the risk of colon and breast cancer. However, the optimal type and volume has not yet been determined. Similarly, there is currently insufficient evidence to establish if physical activity protects against other cancers. Regarding survival, there is some intriguing data suggesting that physical activity performed after diagnosis is associated with lower cancer mortality, but more studies of this type are needed before conclusions can be drawn. The evidence base on rehabilitation is expanding and currently indicates positive effects on outcomes relating to physical functioning and quality of life, both during cancer therapy, and after treatment completion. Research is ongoing in all these areas in the attempt to understand and maximize the benefits of physical activity in relation to cancer.
ACKNOWLEDGMENTS
Clare Stevinson is supported by a Postdoctoral Fellowship from the Faculty of Physical Education and Recreation, University of Alberta. Kerry S. Courneya is supported by the Canada Research Chairs Program and a Research Team Grant from the National Cancer Institute of Canada with funds from the Canadian Cancer Society and the Sociobehavioral Cancer Research Network.
REFERENCES
Courneya KS & Friedenreich CM. (2001). Framework PEACE: An organizational model for examining physical exercise across the cancer experience. Annals of Behavioral Medicine, 23, 263–272.
Holmes M, Chen WY, Feskanich D, Kroenke CH, & Colditz GA. (2005). Physical activity and survival after breast cancer diagnosis. Journal of the American Medical Association, 293, 2479–2486.
Humpel N & Iverson DC. (2005). Review and critique of the quality of exercise recommendations for cancer patients and survivors. Supportive Care in Cancer, 13, 493–502.
International Agency for Research on Cancer. (2002). Weight control and physical activity, IARC handbook of cancer prevention (Vol 6). Lyon, France, IARC Press.
Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, Gansler T, et al. (2006). American cancer society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA A Cancer Journal for Clinicians, 56, 254–281.
McNeely ML, Peddle C, Parliament M, & Courneya KS. (2006b). Cancer rehabilitation: Recommendations for integrating exercise programming in the clinical practice setting. Current Cancer Therapy Reviews, 2, 351–360.
Ness KK, Wall MM, Oakes M, Robison LL, & Gurney JG. (2006). Physical performance limitations and participation restrictions among cancer survivors: A population-based study. Annals of Epidemiology, 16, 197–205.
Rundle A. (2005). Molecular Epidemiology of Physical Activity and Cancer. Cancer Epidemiology, Biomarkers & Prevention, 14, 227–236.
Schmitz KH, Holtzman J, Courneya KS, Mâsse LC, Duval S, & Kane R. (2005). Controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. Cancer Epidemiology, Biomarkers & Prevention, 14, 1588–1595.
Westerlind KC. (2003). Physical activity and cancer prevention—mechanisms. Medicine and Science in Sports and Exercise, 35, 1834–1840.
(A complete reference list for this chapter is available online at http://www.oup.com/us/integrativemedicine).
 Research has established that physical activity plays an important role in the prevention and management of many chronic medical conditions.
Research has established that physical activity plays an important role in the prevention and management of many chronic medical conditions. Physical activity helps to protect against colon and breast cancer, and possibly endometrial, lung, and prostate cancer.
Physical activity helps to protect against colon and breast cancer, and possibly endometrial, lung, and prostate cancer. Physical activity is essential for maintaining a healthy body weight, which is important since obesity is a major risk factor for many cancers.
Physical activity is essential for maintaining a healthy body weight, which is important since obesity is a major risk factor for many cancers. Some preliminary data suggest that postdiagnosis physical activity may be associated with increased survival in colon and breast cancer.
Some preliminary data suggest that postdiagnosis physical activity may be associated with increased survival in colon and breast cancer. Physical activity can improve general health and strength, contributing to enhanced functional status and ability to cope with disease- and treatment-related symptoms and side effects.
Physical activity can improve general health and strength, contributing to enhanced functional status and ability to cope with disease- and treatment-related symptoms and side effects. Current evidence suggests that by adhering to a graded exercise program, cancer survivors can maintain or increase their level of conditioning function and thereby avoid becoming trapped in a perpetuating cycle of deteriorating physical function and increasing fatigue.
Current evidence suggests that by adhering to a graded exercise program, cancer survivors can maintain or increase their level of conditioning function and thereby avoid becoming trapped in a perpetuating cycle of deteriorating physical function and increasing fatigue. Physical activity can also enhance quality of life, and help to prevent other chronic conditions (e.g., diabetes or cardiovascular disease) for which cancer survivors are at increased risk.
Physical activity can also enhance quality of life, and help to prevent other chronic conditions (e.g., diabetes or cardiovascular disease) for which cancer survivors are at increased risk. The risk/benefit profile of physical activity is favorable, assuming that key safety issues are considered for each individual before initiating an exercise program.
The risk/benefit profile of physical activity is favorable, assuming that key safety issues are considered for each individual before initiating an exercise program.










