Introduction
Chelating reagents such as β-hydroxyoximes and β-diketones are excellent for separating and concentrating copper (II) and nickel (II) by solvent extraction from ammoniacal alkaline solutions. Both copper (II) and nickel (II) are usually extracted at the same time, and co-extraction of ammonia is well known to occur [1–10]. The extracted ammonia is removed with a dilute acid, which is followed by the selective stripping of nickel (II) with dilute sulfuric acid and then by stripping of copper (II) with more concentrated sulfuric acid . Because the performance of this system depends on many operational parameters, quantitative modeling of this process is important in order to support its optimization ; however, the studies from this view point are scarce. Recently, using LIX® 84-I , we have studied the equilibrium extraction of ammonia [11], copper (II)-ammonia [12], nickel (II)-ammonia , and copper (II)-nickel (II)-ammonia [13]. As a result, we have established a quantitative model of the extraction part of this ammoniacal process and thus summarize it here.
Experimental
Preparation of Reagents and Solution
The hydroxyoxime reagent LIX® 84-I (active component: 2-hydroxy-5-nonylacetophenone oxime; lot no. 0684I001; Cognis Ireland Ltd.) and the aliphatic solvent ShellSol® D70 (lot no. 50801 and 50713; Shell Japan Ltd.) were used as extractant and diluent without further purification , respectively.
As the feed organic phase, 10 vol.% LIX® 84-I was used, where total hydroxyoxime (HR) concentration, CBO, was 0.16 mol/L as determined by the maximum copper (II) loading. The feed aqueous phases were prepared by mixing the appropriate stock solutions of ammonium salt (nitrate and sulfate), copper (II) and nickel (II) nitrates, and pH adjustment reagent (various concentrations of nitric acid or sodium hydroxide) in a 50-mL centrifuge bottle.
Extraction and Analysis of Ammonia, Cu(II), and Ni(II)
The extraction equilibria were attained in a water bath thermostat at 298 K. The detailed procedure for extraction and the analysis methods for ammonia , copper , nickel , and pH were described elsewhere [11–13].
![$$ D({\text{M}}) = \left[ {\text{M}} \right]_{{{\text{T}},{\text{org}}}} /[{\text{M}}]_{{{\text{T}},{\text{aq}}}} $$](../images/468727_1_En_167_Chapter/468727_1_En_167_Chapter_TeX_Equ1.png)
![$$ D\left( {{\text{NH}}_{ 3} } \right) = \left[ {{\text{NH}}_{ 3} } \right]_{{{\text{T}},{\text{org}}}} /\left( {\left[ {{\text{NH}}_{ 3} } \right]_{{{\text{init}},{\text{T}},{\text{aq}}}} - \left[ {{\text{NH}}_{ 3} } \right]_{{{\text{T}},{\text{org}}}} } \right) $$](../images/468727_1_En_167_Chapter/468727_1_En_167_Chapter_TeX_Equ2.png)
Results and Discussion
Equilibria and their constants considered in our model
Equilibria | Equilibrium constanta | No. | References |
|---|---|---|---|
Aqueous phase | |||
NH4+aq ↔ NH3 aq + Haq+ | pKa(NH4+) = 0.0562I + 9.33 | (3) | [14] |
Maq2+ + m NH3 aq ↔ M(NH3) m2+aq | log βm = ∑1 m (log K m0 + A[NH3]T + B)b | (4) | [15] |
HSO4−aq ↔Haq+ + SO42−aq | ln (Ka(HSO4−)/0.0104) = 5.29I1/2/(1 + 0.56I1/2) | (5) | [16] |
Maq2+ + SO42−aq ↔ MSO4 aq | log β(CuSO4) = 2.099 − 4.05I1/2/(1 + 1.618I1/2) + 0.05201I log β(NiSO4) = 2.10 | (6) | |
Organic phase | |||
(HR)i org + HRorg ↔ (HR)i+1 org | Km = 9.9 | (7) | [14] |
(HR)j·NH3 org + HRorg ↔ (HR)j+1·NH3 org | K2 = 14 | (8) | [12] |
(HR·NH3)k org + HR·NH3 org ↔ (HR·NH3)k+1 org | K3 = 25 | (9) | [12] |
Extraction | |||
NH3 aq + HRorg ↔ HR·NH3 org | K1 = 0.17 | (10) | [12] |
Maq2+ + 2 HRorg ↔ MR2 org + 2 Haq+ | log Ke(Cu) = 0.112I + 1.07 log Ke(Ni) = 0.129I − 4.80 | (11) | |
MR2 aq ↔ MR2 org | log Kd(Cu) = 4.2 log Kd(Ni) = 4.3 | (12) | |
NiR2 org + NH3 aq ↔ NiR2·NH3org | K4 = 0.1 | (13) | [13] |
NiR2 org + 2 NH3 aq ↔ NiR2·(NH3)2 org | K5 = 0.063 | (14) | |
Firstly, we measured the distribution ratios of ammonia using different concentrations of ammonium nitrate solution (1.1–5.3 mol/L) at different equilibrium pH values [11, 12]. The log D(NH3) value at a fixed ammonium nitrate concentration monotonically increased from −3 to −1.8 with pHeq from 8 to 10 and then tended to be constant. At a fixed pHeq (8–10), the log D(NH3) slightly decreased with increasing ammonium nitrate concentration. The results were analyzed assuming the ammonia extraction by HR as the adduct HR·NH3 (Eq. 10), the successive association of the adduct with HR (Eq. 8), and the successive self-association of the adduct (Eq. 9), where acid dissociation of ammonium ion (Eq. 3) and successive association of HR (Eq. 7) both with known equilibrium constants were also considered; that is, the equilibrium constants of K1, K2, and K3 were obtained by a least squares method so that the experimental data satisfied the simultaneous equations of the relevant equilibrium constants and material balances. Although K1 contains the aqueous species (NH3 aq), the K1 value is found to be independent of ionic strength. This would be because NH3 is a neutral species, and thus its activity coefficient is not sensitive to ionic strength in comparison with the ionic species such as Cu2+ and Ni2+ as described below. The slight decrease in D(NH3) with increasing ammonium nitrate concentration would be due to a decrease in Ka(NH4+) as expressed by Eq. (3).

The distribution ratios of M and ammonia in the alkaline region were also measured using 3.2 mol/L ammonium nitrate solution containing 0.05 mol/L M(NO3)2 solution, and the results were compared with the values calculated from the relevant equilibrium constants and material balances assuming the extracted metal complex was only MR2 [12, 13]. The agreement between experiment and calculation for D(Cu) and D(NH3) in the copper extraction was satisfactory. On the other hand, in the nickel extraction , the calculated D(NH3) values were significantly lower than the experimental values, though the calculated D(Ni) values were in good agreement with the experimental values. Thus, the extraction equilibrium constants of ammonia with NiR2, K4 and K5, were obtained by the least squares method so that the experimental data for nickel and ammonia satisfied the simultaneous equations of the relevant equilibrium constants and material balances. As a result, the K4 and K5 values were determined, and good fitting was obtained not only in nickel but also in ammonia .

A similar comparison was also made at different ammonium sulfate concentrations (0.5–2.3 mol/L) with satisfactory agreement. Here, we have established the model to quantitatively calculate the distribution ratios of copper and nickel together with that of ammonia at a given pHeq in sulfate solution.
![$$ \begin{aligned} {\text{pH}} & = 8.11 + 2.33\,[{\text{NaOH}}]_{\text{aq}} - 1.02\,[{\text{NaOH}}]_{\text{aq}}^{2} + 0.21\,[{\text{NaOH}}]_{\text{aq}}^{3} \\ & \quad - 3.34\,[{\text{Cu}}]_{\text{aq}} - 4.29\,[{\text{Ni}}]_{\text{aq}} \\ \end{aligned} $$](../images/468727_1_En_167_Chapter/468727_1_En_167_Chapter_TeX_Equ3.png)
![$$ [{\text{NaOH}}]_{\text{aq}} = [{\text{NaOH}}]_{{{\text{init}},{\text{aq}}}} {-}2\left( {[{\text{Cu}}]_{{{\text{T}},{\text{org}}}} + [{\text{Ni}}]_{{{\text{T,}}\,{\text{org}}}} } \right) $$](../images/468727_1_En_167_Chapter/468727_1_En_167_Chapter_TeX_Equ4.png)
Therefore, by considering the equations for relevant equilibrium constants, material balances, and Eqs. (15) and (16), the distribution ratios can be calculated using initial sodium hydroxide concentration.
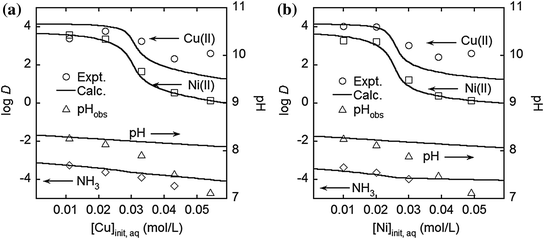
The effect of initial total metal concentration in the aqueous phase on log D(M) for the extraction from the copper -nickel mixed solutions [13]
There is a sharp decrease in D(M) at the metal concentration of the X-axis of 0.02–0.03 mol/L, because, in this region, the total metal concentration in the organic phase is close to the loading capacity of the extractant. The distribution ratios of both metals are very high (>103) when the metal concentration of the X-axis is less than 0.02 mol/L; thus, we can achieve complete extraction of both metals at the same time in this condition. The distribution ratio of ammonia is about 10−4, which corresponds to the ammonia concentration in the organic phase of 5 mg/L; thus, it is easy to scrub loaded ammonia before metal stripping. The curves in Fig. 3 are the predicted values which are basically in good agreement with the experimental data. When pH is less than 8, there are some deviations in the distribution ratios and pH values. This is because the empirical equation of Eq. (15) cannot be applied to this region.
Conclusions
An equilibrium model has been established for quantitative prediction of the extraction behavior of copper , nickel , and ammonia with LIX® 84-I from ammoniacal alkaline solutions. This model will be useful for the design and control of the ammoniacal process for copper and nickel .
Acknowledgements
Mr. S. Wang sincerely thanks the China Scholarship Council for providing his scholarship (201406370020).