Discrete Practice Answers
- D
This is a racemic mixture of 2-butanol because it consists of equimolar amounts of (R)-2-butanol and (S)-2-butanol. The (R)-2-butanol molecule rotates the plane of polarized light in one direction, and the (S)-2-butanol rotates it by the same angle but in the opposite direction; as a result, no net rotation of polarized light is observed. - B
The maximum number of stereoisomers of a compound equals 2n, where n is the number of chiral carbons in the compound. In this molecule, C-1 (the aldehydic carbon) is not chiral, nor is C-5 (because it is attached to two hydrogen atoms). Therefore, with three chiral centers, there are 23 = 8 stereoisomers. - C
This answer choice is an example of a meso compound—a compound that contains chiral centers but has an internal plane of symmetry:
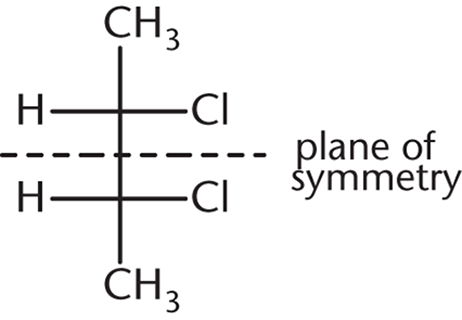
Owing to this internal plane of symmetry, the molecule is achiral and, hence, optically inactive. (A) and (B) are enantiomers of each other and will certainly show optical activity on their own. (D), because it contains a chiral carbon and no internal plane of symmetry, is optically active as well.
- C
To be considered a chiral center, a carbon must have four different substituents. There are eight stereocenters in this molecule, which are marked below with asterisks.
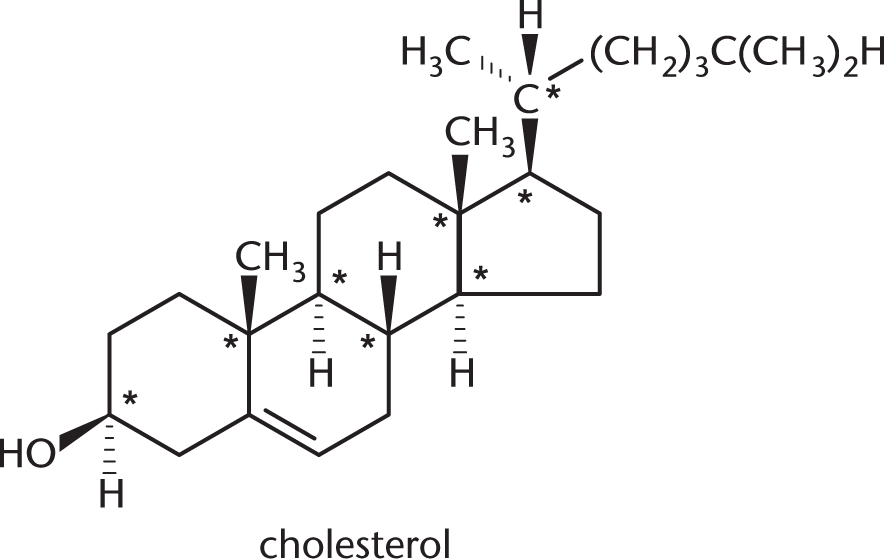
The other carbons are not chiral for various reasons. Many are bonded to two hydrogens; others participate in double bonds, which count as two bonds to the same atom.
- B
This molecule is a chair conformation in which the two equatorial methyl groups are trans to each other. Because the axial methyl hydrogens do not compete for the same space as the hydrogens attached to the ring, this conformation ensures the least amount of steric strain. (A) would be less stable because the diaxial methyl group hydrogens are closer to the hydrogens on the ring, causing greater steric strain. (C) is incorrect because it is in the more unstable boat conformation. - C
The relative configuration is retained because the bonds of the stereocenter are not broken; thus, the positions of groups around the chiral carbon are maintained. The absolute configuration is also retained because both the reactant and product are (R). - A
These compounds are nonsuperimposable mirror images. To make analysis a bit easier, we can rotate structure II 180° to look like structure III. Structures I and III more clearly have opposite stereochemistry at every chiral center, meaning that they are enantiomers.
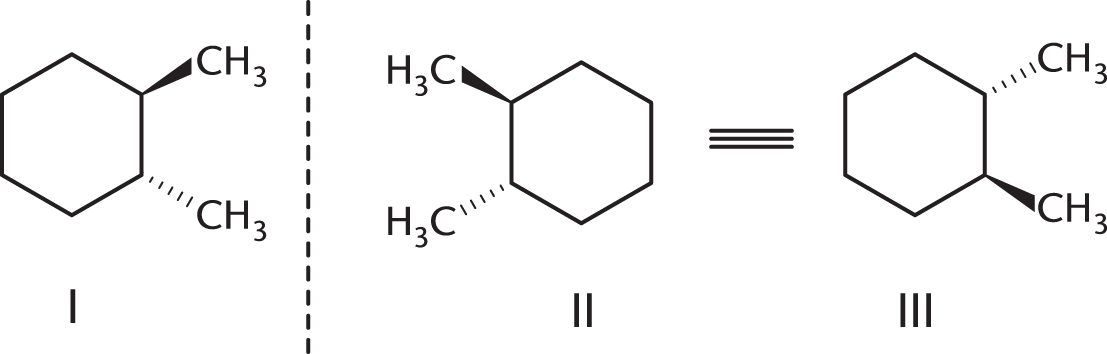
(B) is incorrect because diastereomers are stereoisomers that are not mirror images of each other. (C) is incorrect because meso compounds must contain a plane of symmetry, which neither of these molecules has. (D) is incorrect because structural isomers are compounds with the same molecular formula but different atomic connections. The connectivity in these two molecules is the same, which means that they are stereoisomers, not structural isomers.
- A
(+)-Glyceraldehyde and (–)-glyceraldehyde, or (R)- and (S)-2,3-dihydroxypropanal, are enantiomers. Enantiomers are nonsuperimposable mirror images. Each has only one chiral center (C-2), which has opposite absolute configuration in these two molecules. - C
(E)-2-butene can also be called trans-2-butene; (Z)-2-butene can also be called cis-2-butene. As such, they are cis–trans isomers. Remember that cis–trans isomers are a subtype of diastereomers in which the position of substituents differs about an immovable bond. Diastereomers are molecules that are non-mirror-image stereoisomers (molecules with the same atomic connectivity). These are not enantiomers because they are not mirror images of each other. - C
Because they have the same molecular formula but different atomic connectivity, 3-methylpentane and hexane are constitutional isomers. - B
These two molecules are stereoisomers of one another, but are not nonsuperimposable mirror images. Therefore, they are diastereomers. Note that these molecules differ by at least one, but not all, chiral carbons. - D
Remember that the equation for specific rotation is
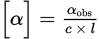 . In this example, αobs is +12° (remember that dextrorotatory, or clockwise, rotation is considered positive), c = 0.5
. In this example, αobs is +12° (remember that dextrorotatory, or clockwise, rotation is considered positive), c = 0.5 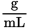 , l = 1 cm = 0.1 dm. Remember that path length is always measured in decimeters when calculating specific rotation. Therefore, the specific rotation can be calculated as:
, l = 1 cm = 0.1 dm. Remember that path length is always measured in decimeters when calculating specific rotation. Therefore, the specific rotation can be calculated as: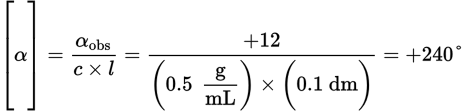
- B
Racemic mixtures like omeprazole contain equimolar amounts of two enantiomers and thus have no observed optical activity. Each of the two enantiomers causes rotation in opposite directions, so their effects cancel out. Esomeprazole only contains one of the two enantiomers and thus should cause rotation of plane-polarized light. - C
Draw out these structures. The two names describe the same molecule, which also happens to be a meso compound because it contains a plane of symmetry. These compounds are not enantiomers because they are superimposable mirror images of one another, not nonsuperimposable mirror images. These compounds are better termed meso-2,3-dihydroxybutanedioic acid:
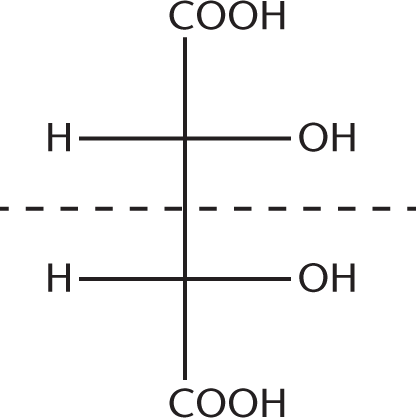
- C
In butane, the position at which the two methyl groups are 120° apart is an eclipsed conformation. This has a moderate amount of energy, although not as high as a totally eclipsed conformation in which the two methyl groups are 0° apart.