3.3 Hybridization
Learning Objectives
After Chapter 3.3, you will be able to:
- Recall the percentage of s character present in a given hybridization level, such as sp2
- Describe the relationship between electron density and resonance structures
- Identify the hybridization of an atom within a complex molecule:
Carbon has the electron configuration 1s22s22p2 and therefore needs four electrons to complete its octet (2s22p6). A typical molecule formed by carbon is methane, CH4. Experimentation shows that the four σ bonds in methane are equivalent. This may seem inconsistent with what we know about
the asymmetrical distribution of carbon’s valence electrons: two electrons in the
2s-orbital, one in the px-orbital, one in the py-orbital, and none in the pz-orbital. This apparent discrepancy is accounted for by the theory of orbital hybridization.
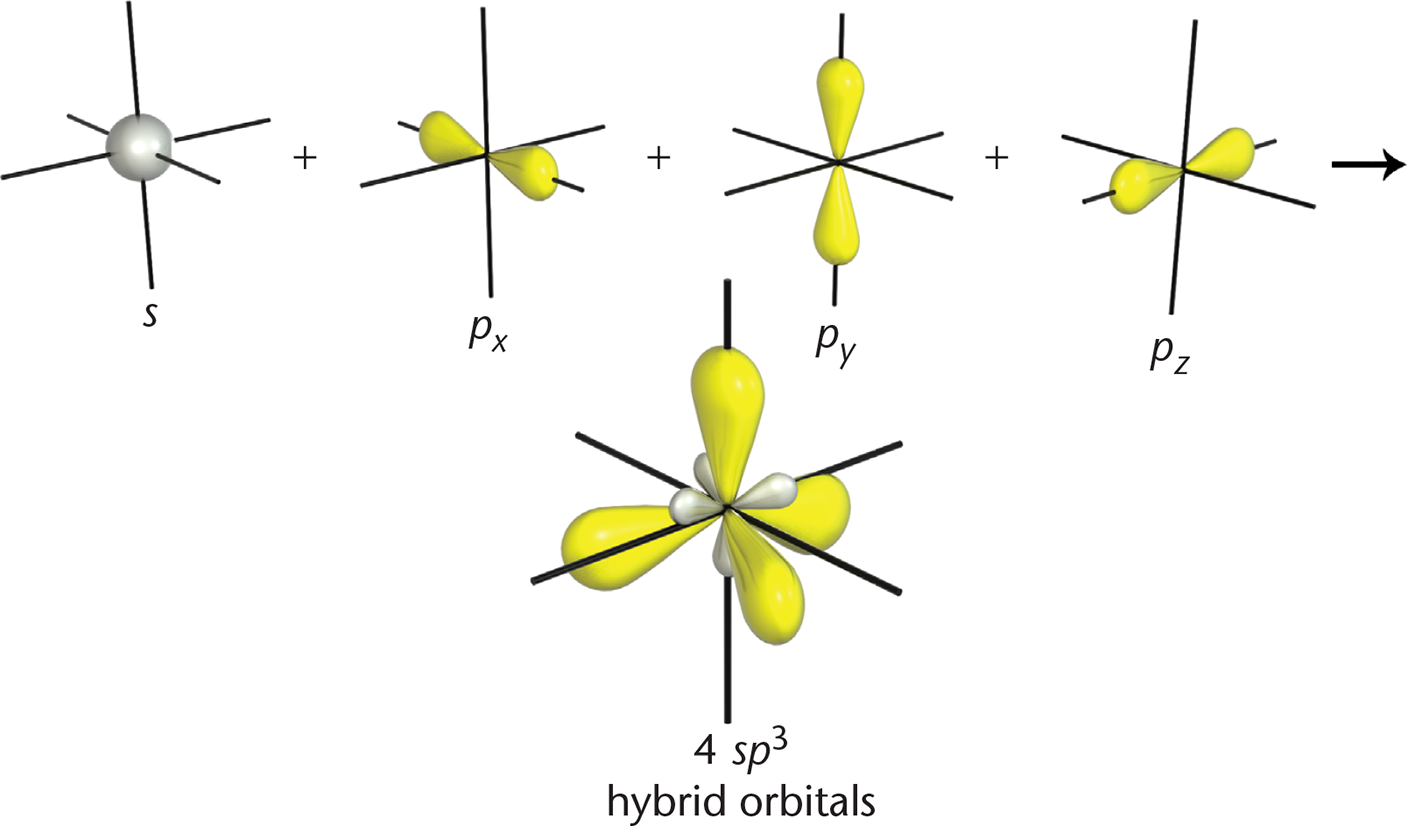
sp3
Hybrid orbitals are formed by mixing different types of orbitals. Just as with molecular orbitals,
we can use advanced mathematics to merge three p-orbitals and one s-orbital. The result? As shown in Figure 3.4, this forms four identical sp3 orbitals with new, hybridized shapes.
All four of these orbitals point toward the vertices of a tetrahedron to minimize
repulsion, which explains why carbon prefers tetrahedral geometry. The hybridization
is accomplished by promoting one of the 2s electrons into the 2pz-orbital, as shown in Figure 3.5. This produces four valence orbitals, each with one
electron, which can be mathematically mixed to model the hybrid orbitals.
Key Concept
Hybridization is a way of making all of the bonds to a central atom equivalent to
each other. The sp3 orbitals are the reason for the tetrahedral shape that is a hallmark of carbon-containing
compounds.
The MCAT sometimes tests how much “s character” a certain hybrid orbital has. To answer such questions, we simply need
to determine what type of hybridization exists and use the name to solve the problem.
For example, in sp3 orbitals, we have one s- and three p-orbitals, so the bond has 25% s character and 75% p character.
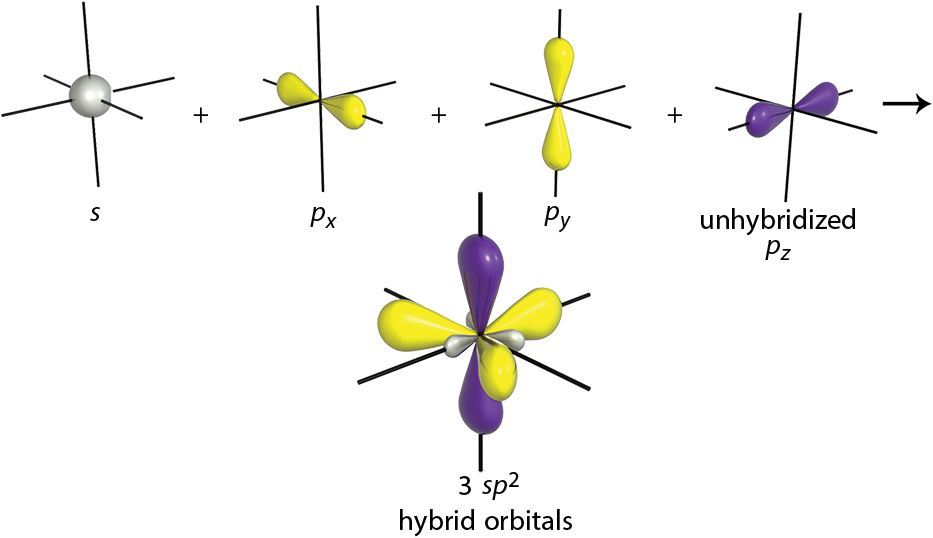
sp2
Although carbon is most often bonded with sp3 hybridization, there are two other possibilities. When one s-orbital is mixed with two p-orbitals, three sp2-hybridized orbitals are formed, as shown in Figure 3.6. These orbitals have 33% s character and 67% p character.
This is the hybridization seen in alkenes. The third p-orbital of each carbon is left unhybridized. This is the orbital that participates
in the π bond. The three sp2 orbitals are oriented 120° apart, which allows for maximum separation. We know that
the unhybridized p-orbital is involved in the π component of the double bond, but what about the hybrid orbitals? In ethene, two
of the sp2 hybridized orbitals will participate in C–H bonds, and the other hybrid orbital will
line up with the π bond and form the σ component of the C=C double bond.
sp
To form a triple bond, we need two of the p-orbitals to form π bonds, and the third p-orbital will combine with the s-orbital to form two sp-orbitals, as shown in Figure 3.7. These orbitals have 50% s character and 50% p character. These orbitals are oriented 180° apart, which explains the linear structure
of molecules containing sp-hybridized carbons. The two π bonds can be between the carbon and one other atom (forming a triple bond, like ethyne),
or between the carbon and two different atoms (forming two double bonds in a row,
like carbon dioxide). In both cases, the molecule is linear about the sp-hybridized carbon.
Resonance
Resonance delocalization of electrons occurs in molecules that have conjugated bonds.
Conjugation requires alternating single and multiple bonds because this pattern aligns a number
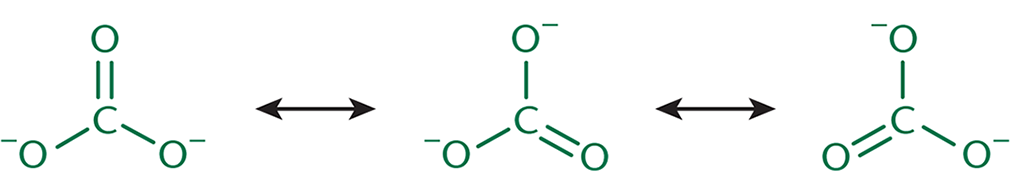
of unhybridized p-orbitals down the backbone of the molecule. π electrons can then delocalize through this p-orbital system, adding stability to the molecule. Resonance structures are drawn
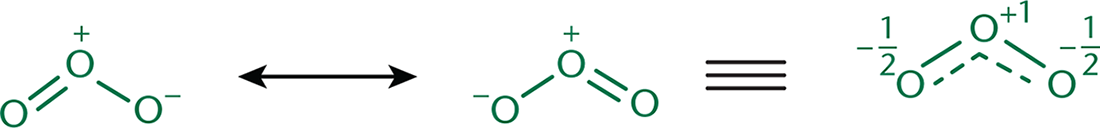
as the various transient forms the molecule takes, as shown in Figure 3.8.
However, these forms aren’t in any sort of equilibrium—the electron density is distributed
throughout, making the true form a hybrid of the resonance structures, as shown with
ozone in Figure 3.9.
If the stability of the various resonance forms differs, then the true electron density
will favor the most stable form. Particular resonance structures can be favored because
they lack formal charges or form full octets on highly electronegative atoms, like
oxygen and nitrogen. Stabilization of positive and negative charges through induction
and aromaticity can also favor certain resonance structures.


 charge.
charge.