
After Chapter 4.1, you will be able to:
In an acid–base reaction, an acid and a base react, resulting in the formation of the conjugate base of the acid and the conjugate acid of the base. This reaction proceeds so long as the reactants are more reactive, or stronger, than the products that they form. We will discuss acid and base definitions and strength in the following section. For the MCAT, we will concern ourselves with the broader Lewis and Brønsted–Lowry definitions of acids and bases. The Lewis definition concerns itself with the transfer of electrons in the formation of coordinate covalent bonds; the Brønsted–Lowry definition focuses on proton transfer.
An acid–base reaction will only proceed if the products that will be formed (the conjugate base of the acid and the conjugate acid of the base) are weaker than the original reactants.
A Lewis acid is defined as an electron acceptor in the formation of a covalent bond. Lewis acids also tend to be electrophiles, which we will touch on in the next section. Lewis acids have vacant p-orbitals into which they can accept an electron pair, or are positively polarized atoms.
Acids and bases are critically important material in organic chemistry, biochemistry, and general chemistry. The most extensive coverage of acids and bases is in Chapter 10 of MCAT General Chemistry Review.
A Lewis base is defined as an electron donor in the formation of a covalent bond. Lewis bases also tend to be nucleophiles, which we will touch on in the next section. Lewis bases have a lone pair of electrons that can be donated, and are often anions, carrying a negative charge.
When Lewis acids and bases interact, they form coordinate covalent bonds—covalent bonds in which both electrons in the bond came from the same starting atom (the Lewis base), as shown in Figure 4.1.

In the Brønsted–Lowry definition, an acid is a species that can donate a proton (H+); a base is a species that can accept a proton. Some molecules, like water, have
the ability to act as either Brønsted–Lowry acids or bases, making them amphoteric. Water can act as an acid by donating its proton to a base, and thus becoming its
conjugate base, OH–. However, water can also act as a base by accepting a proton from an acid to become
its conjugate acid, H3O+. The degree to which a molecule acts as an acid or a base is dependent upon the properties
of the solution—water can only act as a base in an acidic solution, and only as an
acid in a basic solution. Other examples of amphoteric molecules include Al(OH)3,
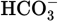 , and
, and
 .
.
The acid dissociation constant, or Ka, measures the strength of an acid in solution. In the dissociation of an acid HA (HA ⇌ H+ + A–), the equilibrium constant is given by:

Equation 4.1
and the pKa can be calculated as:
pKa = –log Ka
Equation 4.2
Thus, more acidic molecules will have a smaller (or even negative) pKa; more basic molecules will have a larger pKa. Acids with a pKa below –2 are considered strong acids, which almost always dissociate completely in aqueous solution. Weak organic acids often have pKa values between –2 and 20. pKa values for common functional groups are shown in Table 4.1.

Generally, bond strength decreases down the periodic table, and acidity therefore increases. Also, the more electronegative an atom, the higher the acidity. When these two trends oppose each other, low bond strength takes precedence.
For the common functional groups on the MCAT, the α-hydrogens of carbonyl compounds deserve special note. α-hydrogens are connected to the α-carbon, which is a carbon adjacent to the carbonyl. Because the enol form of carbonyl-containing carbanions is stabilized by resonance, these are acidic hydrogens that are easily lost. We will go into greater depth about enolate chemistry in Chapters 7 through 9 of MCAT Organic Chemistry Review.
We can also apply these acid and base rules directly to the functional groups that appear on the MCAT. Functional groups that act as acids include alcohols, aldehydes and ketones (at the α-carbon), carboxylic acids, and most carboxylic acid derivatives. These compounds are therefore easier to target with basic (or nucleophilic) reactants because they readily accept a lone pair.
Amines and amides are the main functional groups that act as bases—keep an eye out for these compounds in the formation of peptide bonds. The nitrogen atom of an amine can form coordinate covalent bonds by donating a lone pair to a Lewis acid.