After Chapter 4.5, you will be able to:
With all of these rules in hand, we can now apply our knowledge in a systematic way to simplify organic chemistry reactions that appear on the MCAT. These steps are described below.
While studying organic chemistry for the MCAT, don’t permit yourself to simply nod along with the reaction mechanisms—get involved! During each step of a mechanism, ask yourself how the trends and problem-solving steps in this chapter play out.
Before you can even start to understand what reactions will occur and what products will form, it is vital to know which compounds IUPAC and common names refer to! If you’re still having trouble with nomenclature, be sure to review Chapter 1 of MCAT Organic Chemistry Review.
Look at the organic molecules in the reaction. What functional groups are in the molecules? Do these functional groups act as acids or bases? How oxidized is the carbon? Are there functional groups that act as good nucleophiles, electrophiles, or leaving groups? This step will help define a category of reactions that can occur with the given functional groups.
In this step, determine the properties of the other reagents in the reaction. Are they acidic or basic? Are they suggestive of a particular reaction? Are they good nucleophiles or a specific solvent? Are they good oxidizing or reducing agents?
Once you’ve identified the functional groups in the compound and the other reagents present, this step should be relatively quick. Remember that more oxidized carbons tend to be more reactive to both nucleophile–electrophile reactions and oxidation–reduction reactions. Note the presence of protecting groups that exist to prevent a particular functional group from reacting.
If the reaction involves an acid or a base, the first step will usually be protonation or deprotonation. If the reaction involves a nucleophile, the first step is generally for the nucleophile to attack the electrophile, forming a bond with it. If the reaction involves an oxidizing or reducing agent, the most oxidized functional group will be oxidized or reduced, accordingly.
Once you know what will react, think through how the reaction will go. Did the protonation or deprotonation of a functional group increase its reactivity? When the nucleophile attacks, how does the carbon respond to avoid having five bonds? Does a leaving group leave, or does a double bond get reduced to a single bond (like the opening of a carbonyl)?
Though not all reactions are stereospecific or stereoselective, these possibilities should be considered when predicting products. For stereospecificity, consider whether the configuration of the reactant necessarily leads to a specific configuration in the product, as seen in SN2 reactions. Stereoselectivity, on the other hand, occurs in reactions where one configuration of product is more readily formed due to product characteristics. Stereoselectivity is seen in many reactions, as different products often possess different traits which affect their relative stability. If there is more than one product, the major product will generally be determined by differences in strain or stability between the two molecules. More strained molecules (with significant angle, torsional, or nonbonded strain) are less likely to form than molecules without significant sources of strain. Products with conjugation (alternating single and multiple bonds) are significantly more stable than those without.
Now, we’ll apply these rules to three novel reactions. Focus on the decision-making element of this process so that you will be able to apply the same logic to reactions that appear on Test Day.
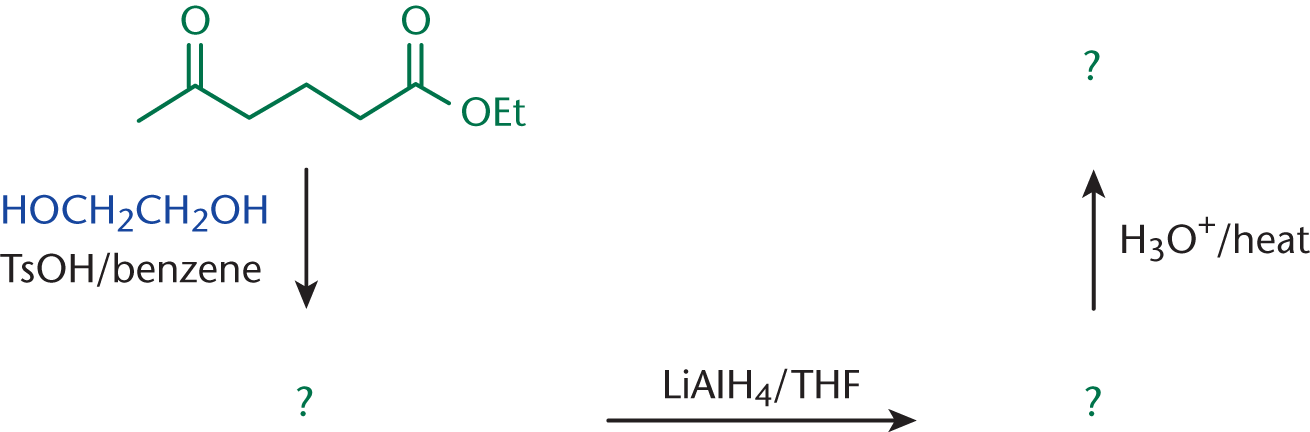
We’ll start with a series of reactions involving ethyl 5-oxohexanoate. First, it is reacted with 1,2-ethanediol and p-toluenesulfonic acid in benzene; second, with lithium aluminum hydride in tetrahydrofuran, followed by a heated acidic workup. What are the intermediates and final product?
Let’s go through the steps:

Let’s see what we came up with. The first intermediate will have a protective diether at the ketone carbonyl. The second will show the reduction of the ester to an alcohol, with the protecting group still present. The third will be our final product.
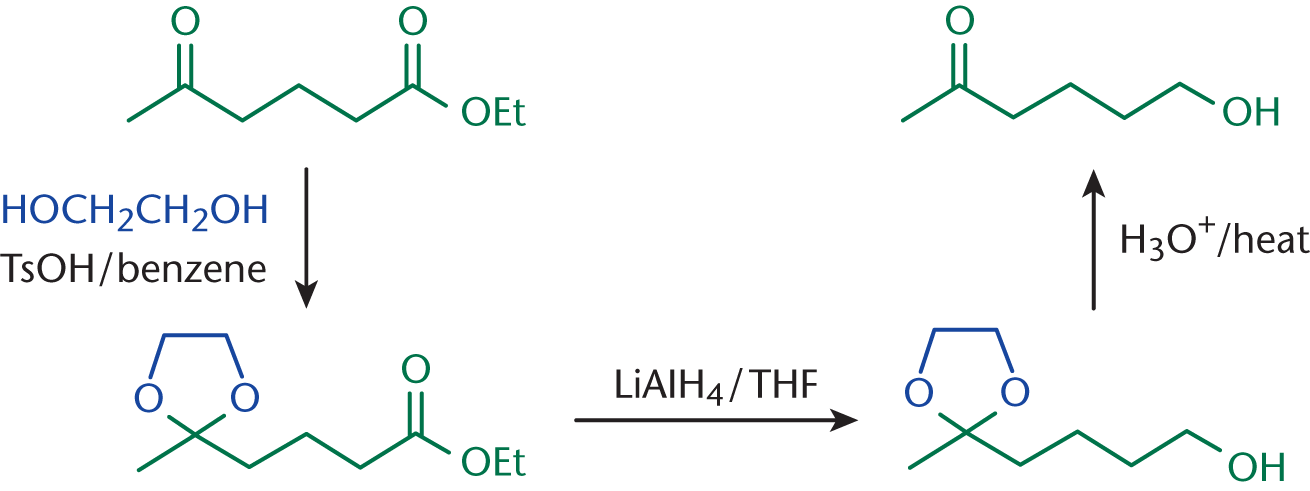
If ethanol is reacted in acidic solution with potassium dichromate, what will the end product be?
Let’s go through the steps again.
Therefore, the primary product of this reaction will be ethanoic acid.
Determine the product of a reaction between 2-amino-3-hydroxypropanoic acid and 2,6-diaminohexanoic acid in aqueous solution.
Let’s go through the steps one last time.
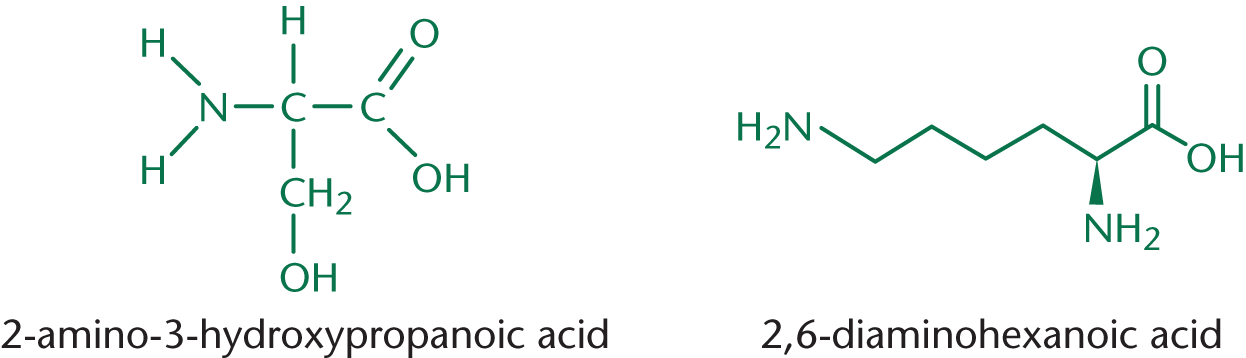
Does this reaction look familiar? It should! 2-amino-3-hydroxypropanoic acid and 2,6-diaminohexanoic acid are serine and lysine, respectively—in this reaction, we are forming a peptide bond. If we treat them as generic amino acids, this is the reaction:

We’ve worked through a few problems here to get a handle on how to use this method. Once you have read further chapters and learned specific mechanisms that we did not touch on here, be sure to come back and see how these rules apply to novel reactions.