Learning Objectives
After Chapter 5.3, you will be able to:
- Recall the process for production of quinones and hydroxyquinones
-
Identify the properties of ubiquinone that allow it to function as an electron carrier:
After Chapter 5.3, you will be able to:

Reactions of phenols proceed in similar fashion to reactions of alcohols. However, as discussed previously, the hydrogen in the hydroxyl group of phenols is particularly acidic because the oxygen-containing anion is resonance-stabilized by the ring.
Treatment of phenols with oxidizing agents produces compounds called quinones (2,5-cyclohexadiene-1,4-diones), as shown in Figure 5.12.
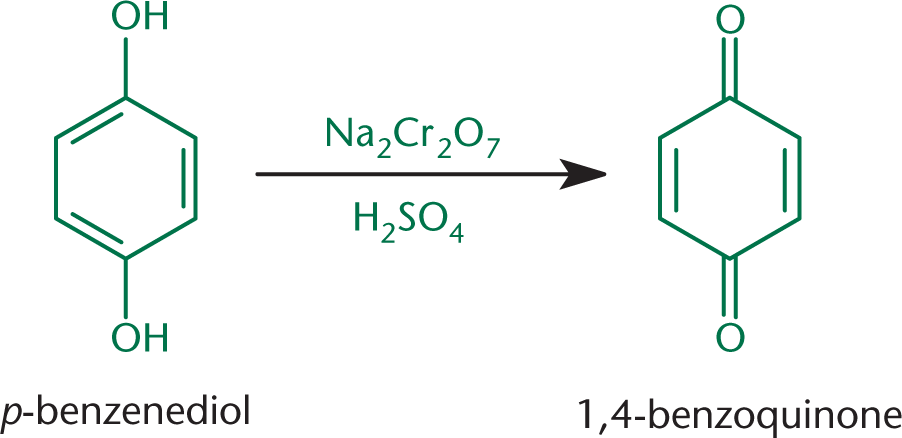
Quinones are named by indicating the position of the carbonyls numerically and adding quinone to the name of the parent phenol. Due to the conjugated ring system, these molecules are resonance-stabilized electrophiles. Remember, however, that these are not necessarily aromatic because they lack the classic aromatic conjugated ring structure. Some quinones do have an aromatic ring, but this is not always the case. Quinones serve as electron acceptors biochemically, specifically in the electron transport chain in both photosynthesis and aerobic respiration. Vitamin K1 is the common name of the quinone 2-methyl-3-[(2E)-3,7,11,15-tetramethylhexadec-2-en-1-yl]naphthoquinone, shown in Figure 5.13. This molecule is also called phylloquinone and is important for photosynthesis and the carboxylation of some of the clotting factors in blood. Vitamin K2, similarly, corresponds to a class of molecules called menaquinones.

Phylloquinone and menaquinone are the common names of Vitamin K1 and Vitamin K2, respectively. These molecules are fat-soluble vitamins that play a role in carboxylation of clotting factors II, VII, IX, and X, and proteins C and S in blood. The functions of fat-soluble vitamins are explored in Chapter 5 of MCAT Biochemistry Review.
These molecules can be further oxidized to form a class of molecules called hydroxyquinones. Hydroxyquinones share the same ring and carbonyl backbone as quinones, but differ by the addition of one or more hydroxyl groups. Many hydroxyquinones have biological activity, and some are used in the synthesis of medications. One classic example is shown in Figure 5.14.
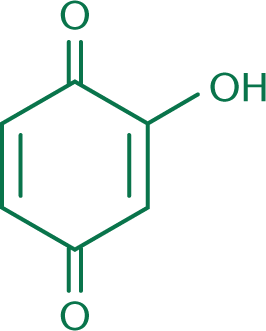
Note the subtle difference in terminology between Figure 5.12 and Figure 5.14. A hydroquinone is a benzene ring with two hydroxyl groups. A hydroxyquinone contains two carbonyls and a variable number of hydroxyl groups.
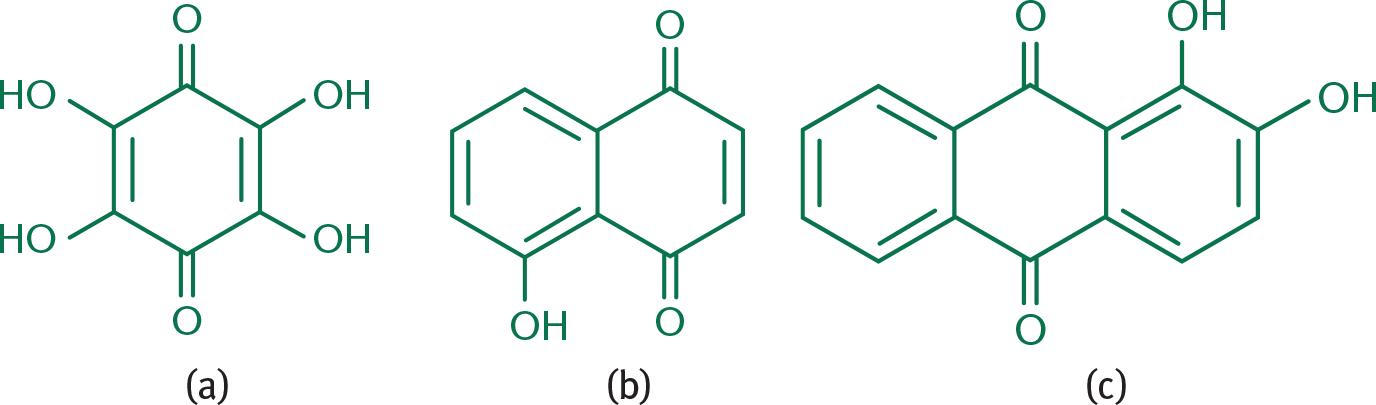
Because of resonance, hydroxyquinones behave like quinones with electron-donating groups, making these slightly less electrophilic (although still quite reactive). When naming these compounds, the position of the hydroxyl groups is indicated by a number, and the total number of hydroxyl groups (if there is more than one) is indicated by a prefix (such as di–, or tri–) with the substituent name hydroxy–. Several examples are shown in Figure 5.15.
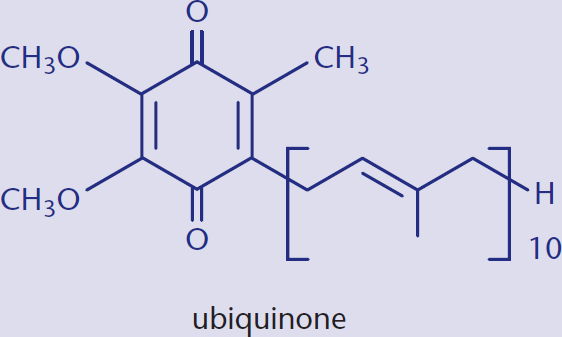
Ubiquinone is one example of a biologically active quinone. Ubiquinone is also called coenzyme Q and is a vital electron carrier associated with Complexes I, II, and III of the electron transport chain. Ubiquinone is the most oxidized form that this molecule takes physiologically: it can also be reduced to ubiquinol upon the acceptance of electrons, as shown in Figure 5.16. This oxidation–reduction capacity allows the molecule to perform its physiological function of electron transport.
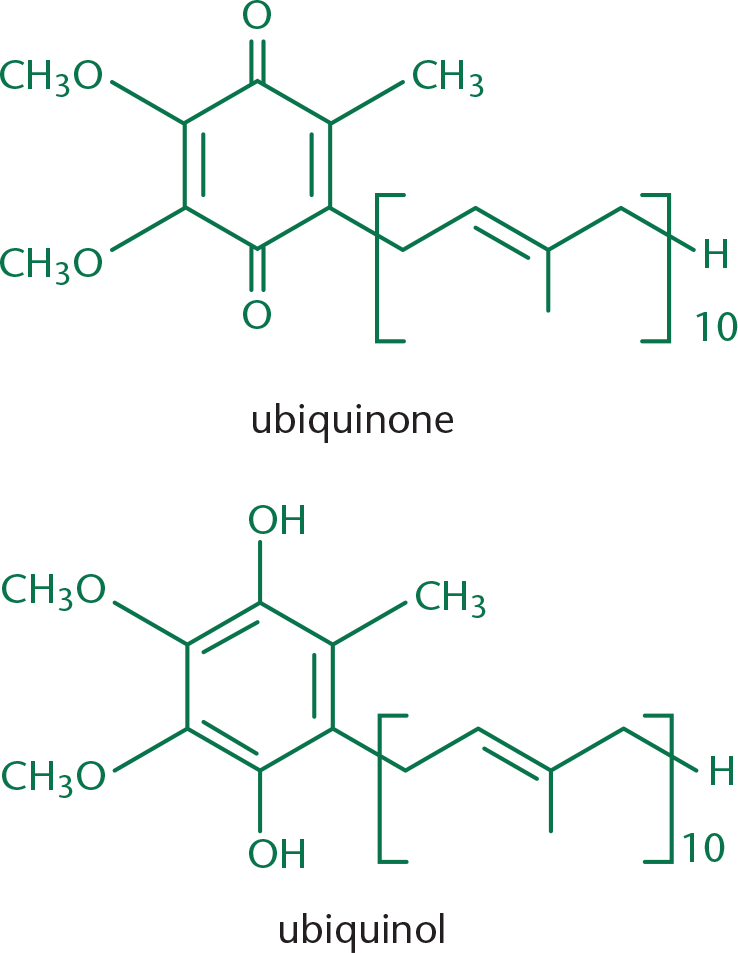
Coenzyme Q plays a role in Complexes I, II, and III of the electron transport chain. In Complex III, it is the main player in the Q cycle, which contributes to the formation of the proton-motive force across the inner mitochondrial membrane. The respiratory complexes are discussed in Chapter 10 of MCAT Biochemistry Review.
The long alkyl chain of this molecule allows it to be lipid soluble, which allows it to act as an electron carrier within the phospholipid bilayer.
Other biological molecules that undergo oxidation–reduction reactions as part of their normal function include NADH, FADH2, and NADPH. These molecules accept and donate electrons readily, similar to ubiquinone, and are discussed more thoroughly in Chapters 9 and 10 of MCAT Biochemistry Review.