After Chapter 6.1, you will be able to:
A ketone has two alkyl groups bonded to the carbonyl, whereas an aldehyde has one alkyl group and one hydrogen. This means that the carbonyl in a ketone is never a terminal group, whereas it always is in an aldehyde. Like many organic compounds, aldehydes and ketones are often strong-smelling compounds. Volatile carbonyls are found in many spices, including cinnamon (cinnamaldehyde), vanilla (vanillin), cumin (cuminaldehyde), dill (carvenone), and ginger (zingerone).
An aldehyde is a terminal functional group. A ketone, on the other hand, will always be internal and can never be a terminal functional group.
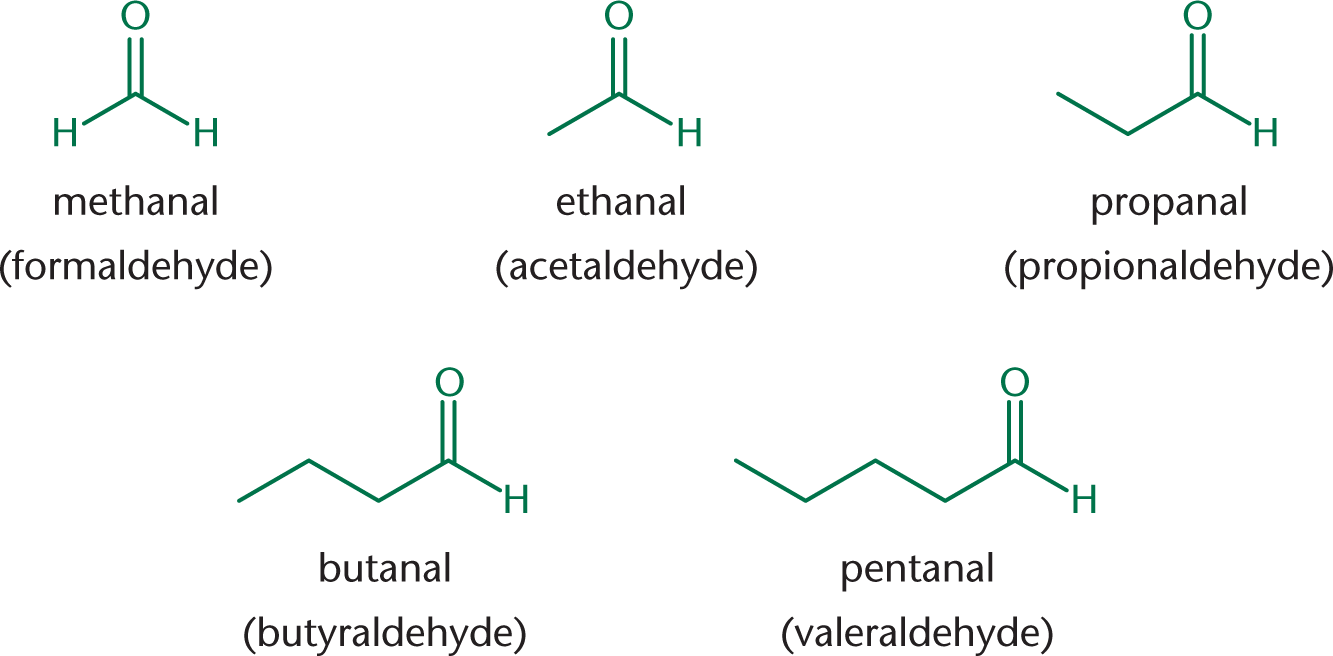
Aldehydes are named by replacing the –e at the end of the alkane name with the suffix –al. Common names for the first five aldehydes, shown in Figure 6.1, are formaldehyde, acetaldehyde, propionaldehyde, butyraldehyde, and valeraldehyde. When aldehydes are named as substituents, use the prefix oxo–.

Notice that these common names have a pattern that can help us: form– will also be seen in formic acid (a one-carbon carboxylic acid), and acet– is seen in many two-carbon compounds (acetylene, acetic acid, and acetyl-CoA).
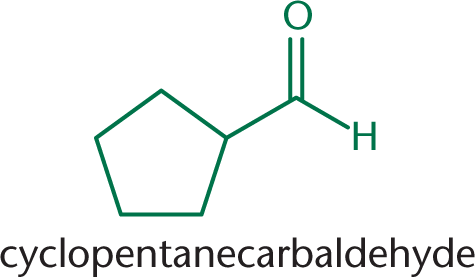
If the aldehyde is attached to a ring, the suffix –carbaldehyde is used instead. This is shown in Figure 6.2.
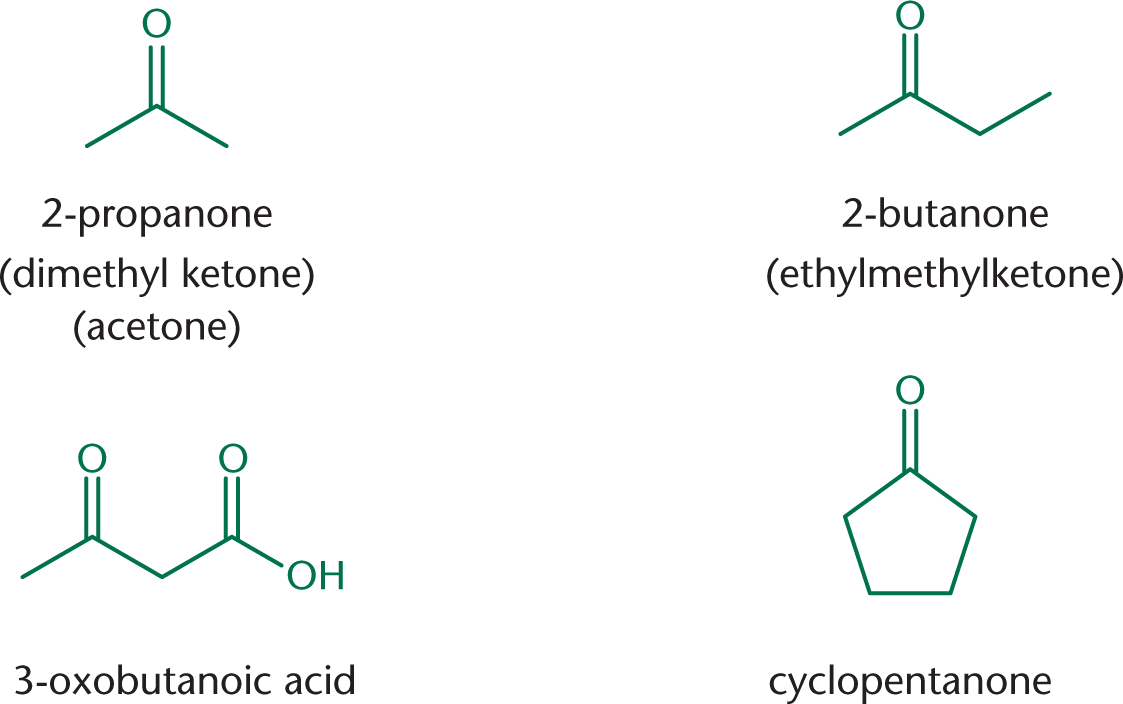
Ketones are named by replacing the –e with the suffix –one. When naming ketones by their common names, the two alkyl groups are named alphabetically, followed by –ketone. When ketones are named as substituents, use either the prefix oxo– or keto–. Figure 6.3 shows some examples of ketones.
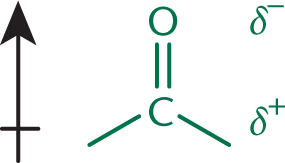
The physical properties of aldehydes and ketones are governed by the presence of the carbonyl group. The dipole of the carbonyl is stronger than the dipole of an alcohol because the double-bonded oxygen is more electron-withdrawing than the single bond to oxygen in the hydroxyl group. In solution, the dipole moments associated with these polar carbonyl groups increase intermolecular attractions, causing an elevation in boiling point relative to their parent alkanes. However, even though aldehydes and ketones have dipoles more polar than those of alcohols, the elevation in boiling point is less than that in alcohols because no hydrogen bonding is present. In reactions, aldehydes and ketones both act as electrophiles, making good targets for nucleophiles. This is due to the electron-withdrawing properties of the carbonyl oxygen, which leaves a partial positive charge on the carbon, as shown in Figure 6.4. Generally, aldehydes are more reactive toward nucleophiles than ketones because they have less steric hindrance and fewer electron-donating alkyl groups.
While the dipole moment in the carbonyl group increases the intermolecular forces (and therefore boiling points) of aldehydes and ketones relative to alkanes, this is not as significant as the impact of hydrogen bonding seen in alcohols.
The carbonyl carbon is the most common electrophile you’ll see on Test Day. Remember why this group has a dipole moment: oxygen is more electronegative and pulls electrons away from the carbon, making the carbon electrophilic and a good target for nucleophiles.
Aldehydes and ketones can be produced by several mechanisms. An aldehyde can be obtained from the partial oxidation of a primary alcohol, although only by pyridinium chlorochromate (PCC; C5H5NH[CrO3Cl]). With any stronger oxidants, aldehydes will continue to be oxidized to carboxylic acids. A ketone can be obtained from the oxidation of a secondary alcohol. This can be performed using reagents ranging from sodium or potassium dichromate salts (Na2Cr2O7 or K2Cr2O7) to chromium trioxide (CrO3) to PCC. When oxidizing a secondary alcohol, there is no concern for oxidizing too far because the reaction will stop at the ketone stage.