After Chapter 10.2, you will be able to:
Synthesis of amino acids occurs by an astonishing variety of mechanisms in vivo. In the lab, several simple reaction mechanisms are exploited to make amino acids neatly and efficiently.
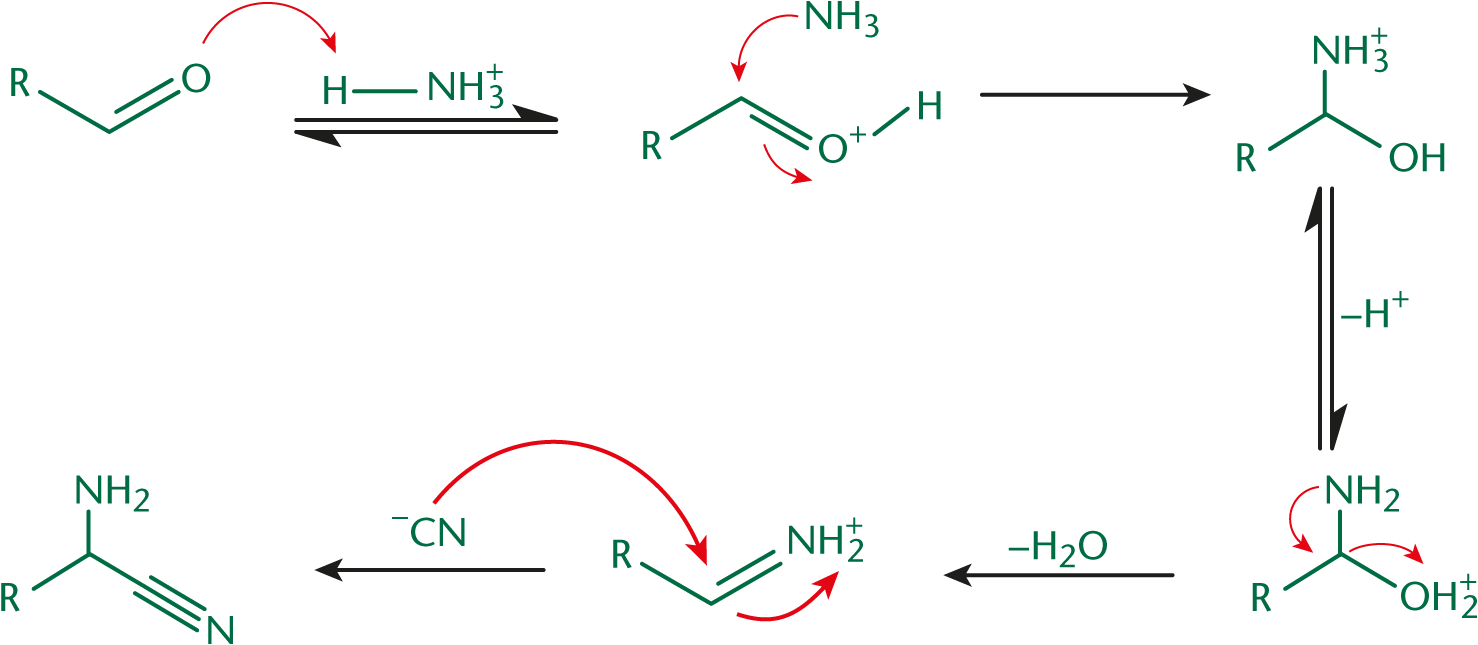
In the Strecker synthesis, one starts with an aldehyde, ammonium chloride (NH4Cl), and potassium cyanide (KCN), as shown in Figure 10.6. The carbonyl oxygen is protonated, increasing the electrophilicity of the carbonyl carbon. Then, as seen in Chapter 6 of MCAT Organic Chemistry Review, ammonia can attack the carbonyl carbon, forming an imine. The imine carbon is also susceptible to nucleophilic addition reactions; thus, the CN– anion from KCN attacks, forming a nitrile group (–C≡N). The final molecule at the end of Step 1 is an aminonitrile—a compound containing an amino group (–NH2) and a nitrile group.
Nitriles have a triple bond between nitrogen and carbon.

In Step 2, the nitrile nitrogen is protonated, increasing the electrophilicity of the nitrile carbon. This is similar to protonating the oxygen of a carbonyl. A water molecule attacks, leading to the creation of a molecule with both imine and hydroxyl moieties on the same carbon. This imine is attacked by another equivalent of water. A carbonyl is formed, kicking off ammonia and creating the carboxylic acid functionality. This step, shown in Figure 10.7, is performed in aqueous acid and can be accelerated by the use of heat.
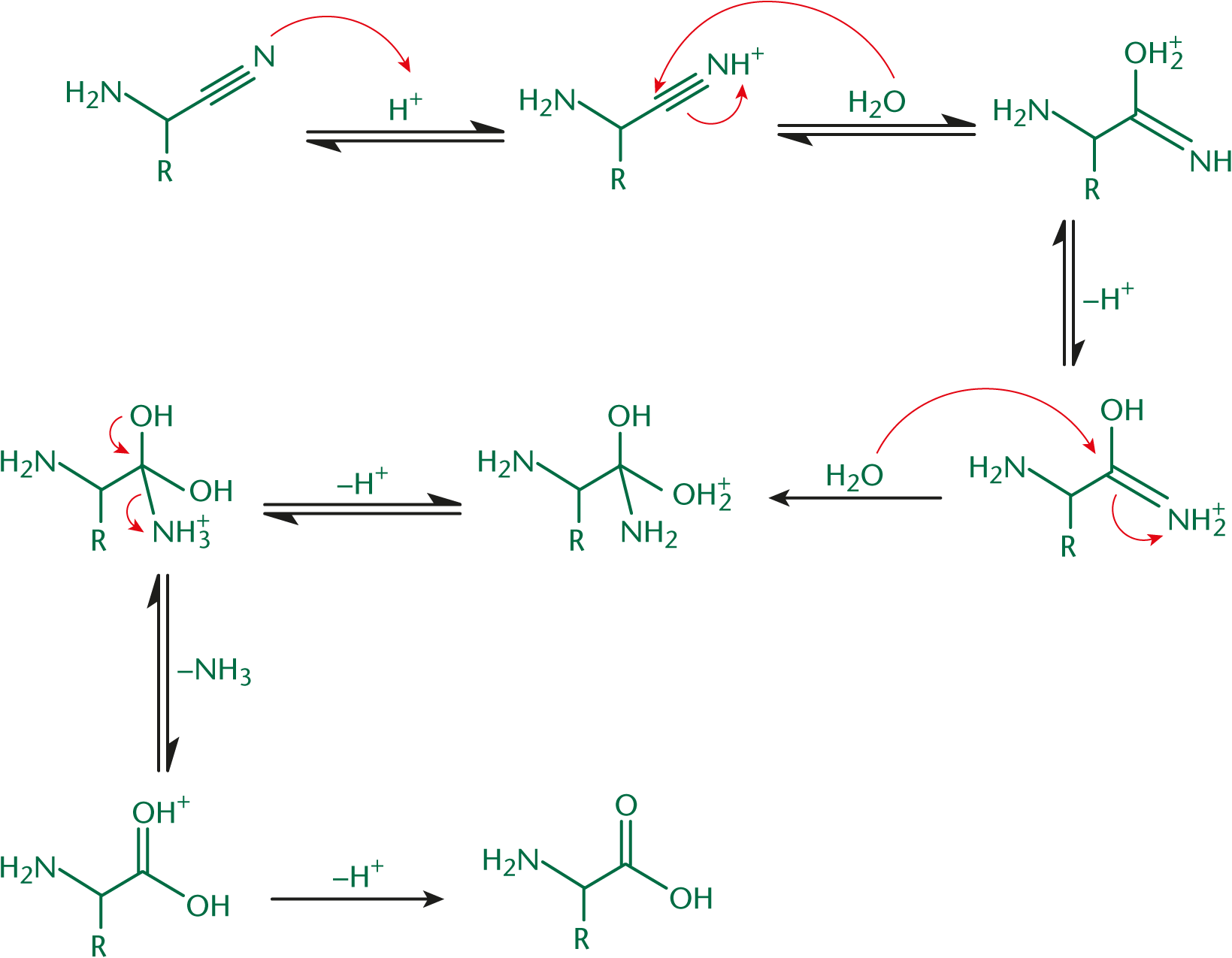
The starting material for the Strecker synthesis is a planar carbonyl-containing compound; therefore, the product of this pathway is a racemic mixture. The incoming nucleophiles are equally able to attack from either side of the carbonyl; thus, both L- and D-amino acids can be generated through this process.
Another way of synthesizing amino acids is the Gabriel (malonic-ester) synthesis, shown in Figure 10.8.
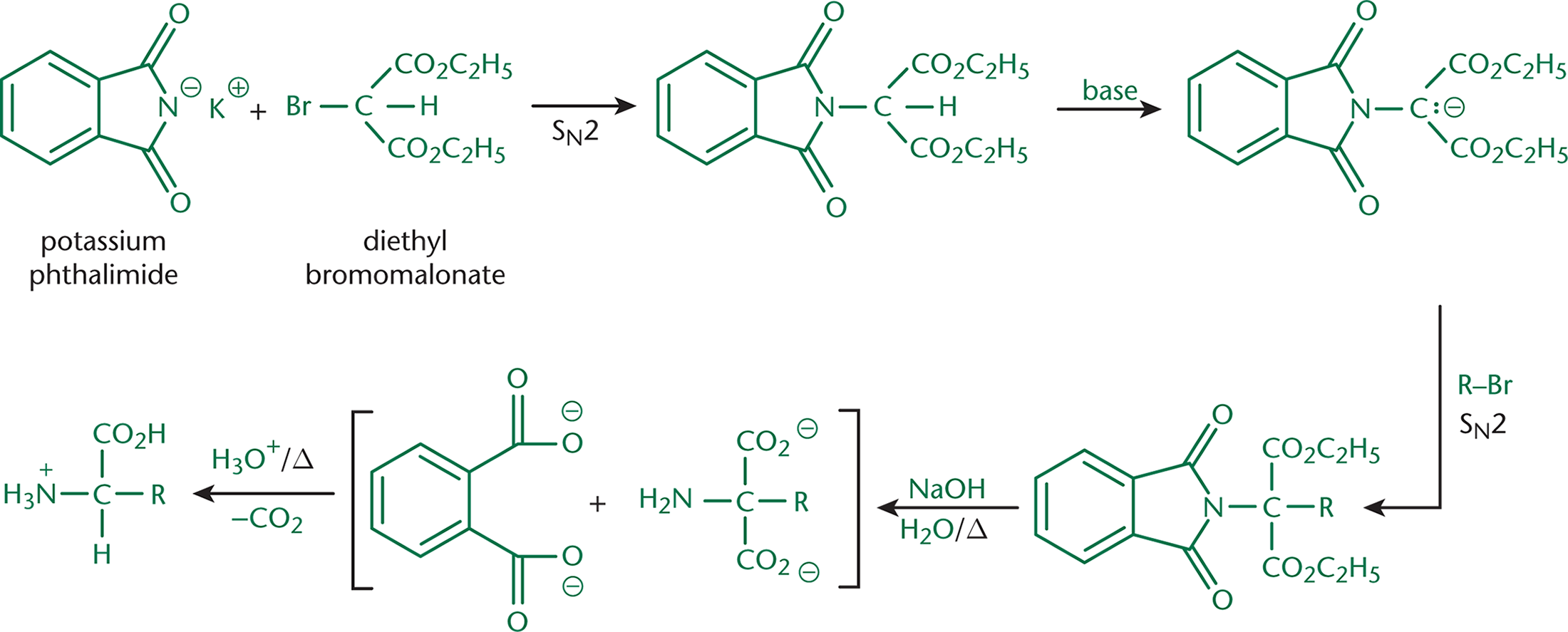
In this method, potassium phthalimide is reacted with diethyl bromomalonate. Phthalimide is acidic and exists in solution as a nucleophilic anion. Diethyl bromomalonate contains a secondary carbon bonded to bromine, a good leaving group. This setup should sound much like the SN2 reactions discussed in Chapter 4 of MCAT Organic Chemistry Review. With phthalimide as the nucleophile, the (secondary) substrate carbon as the electrophile, and bromine as the leaving group, this reaction generates a phthalimidomalonic ester. Consider the benefits of using such a large nucleophile. The bulkiness of this group creates steric hindrance, which prevents the substrate carbon from undergoing multiple substitutions.
Instead, in the presence of base, this carbon (which is the α-carbon between two carbonyls) can easily be deprotonated. The molecule as a whole can then act as a nucleophile, attacking the substrate carbon of a bromoalkane. This is another example of an SN2 reaction. The nucleophile is the large, deprotonated phthalimidomalonic ester, the electrophile is the substrate carbon, and the leaving group is the bromide anion.
Next, this molecule is hydrolyzed with strong base and heat. Much like converting a cyclic anhydride into a dioic acid, the phthalimide moiety is removed as phthalic acid (with two carboxylic acids). The malonic ester is hydrolyzed to a dicarboxylic acid with an amine on the α-carbon.
Finally, this dicarboxylic acid, which is a 1,3-dicarbonyl, can be decarboxylated through the addition of acid and heat. The loss of a molecule of carbon dioxide results in the formation of the complete amino acid.
Like the Strecker synthesis, the Gabriel synthesis starts with a planar molecule; thus, the product is a racemic mixture of L- and D-amino acids.
Both the Strecker and Gabriel synthesis methods result in a racemic mixture of amino acids.