Discrete Practice Questions
-
Which of the following amino acids does not have an L-enantiomer?
- Cysteine
- Threonine
- Glutamic acid
- Glycine
-
Which of the following would be formed if methyl bromide were reacted with phthalimide and followed by hydrolysis with an aqueous base?
- C2H5NH2
- CH3NH2
- (C2H5)3N
- (CH3)4N+Br–
-
Which of the following amino acids contain(s) sulfur?
- Cysteine
- Serine
- Methionine
- I only
- I and III only
- II and III only
- I, II, and III
-
Nylon, a polyamide, is produced from hexanediamine and a substance, X. This substance, X, is most probably a(n):
- amine.
- carboxylic acid.
- ketone.
- alcohol.
-
Intermediates in the Strecker synthesis include all of the following nitrogen-containing functional groups EXCEPT a(n):
- nitrile.
- imine.
- amide.
- amine.
-
A biochemist is synthesizing valine, shown below, using the Strecker synthesis. Which of the following carbonyl-containing compounds would be an appropriate starting reactant in this synthesis?
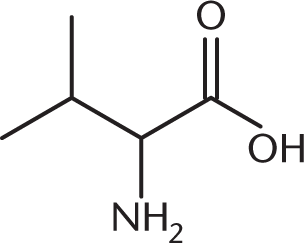
- 2-Propanone
- Propanal
- 2-Methylpropanal
- Butanal
-
Why is the C–N bond of an amide planar?
- It has partial double-bond character.
- It is sp3-hybridized.
- It has some sp2 character.
- I only
- II only
- I and II only
- I and III only
-
Which of the primary methods of amino acid synthesis results in an optically active solution?
- The Strecker synthesis only
- The Gabriel synthesis only
- Both the Strecker and Gabriel syntheses
- Neither the Strecker nor the Gabriel syntheses
-
During the Gabriel synthesis, phthalimide serves as the:
- nucleophile.
- base.
- leaving group.
- electrophile.
-
Each of the following reaction types occurs during the Gabriel synthesis EXCEPT:
- decarboxylation.
- nucleophilic substitution.
- dehydration.
- hydrolysis.
-
At physiological pH, which two forms of phosphoric acid have the highest concentrations?
- H3PO4 and

-
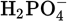 and
and

-
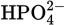 and
and
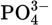
-
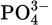 and H3PO4
and H3PO4
- H3PO4 and
-
In aqueous solution, pyrophosphate will likely:
- form insoluble complexes.
- be stable and inert.
- degrade into inorganic phosphate.
- decrease the polarity of the solvent.
-
What would be the charge of aspartic acid at pH 7?
- Neutral
- Negative
- Positive
- There is not enough information to answer the question.
-
When a bond is created between two nucleotide triphosphates in DNA synthesis, the small molecule released from this reaction is:
- pyrophosphate.
- inorganic phosphate.
- ATP.
- organic phosphate.
-
The hydrogens of phosphoric acid have pKa values that:
- allow high buffering capacity over a small pH range.
- allow moderate buffering capacity over a large pH range.
- allow low buffering capacity over a small pH range.
- do not allow buffering.