Discrete Practice Answers
- D
Glycine’s R group is a hydrogen atom; this amino acid is therefore achiral because the central carbon is not bonded to four different substituents. The other amino acids are all chiral and therefore have both L- and D-enantiomers. - B
This reaction is similar to the Gabriel synthesis. Phthalimide acts as a nucleophile, the methyl carbon acts as an electrophile, and bromide acts as the leaving group. Therefore, the reaction between methyl bromide and phthalimide results in the formation of methyl phthalimide. Subsequent hydrolysis then yields methylamine. - B
Cysteine is well known for containing a sulfur atom because it is able to form disulfide bridges; however, methionine also contains a sulfur atom in its R group. - B
An amide is formed from an amine and a carboxyl group or its acyl derivatives. In this question, an amine is already given; the compound to be identified must be an acyl compound. The only acyl compound among the choices given is a carboxylic acid. - C
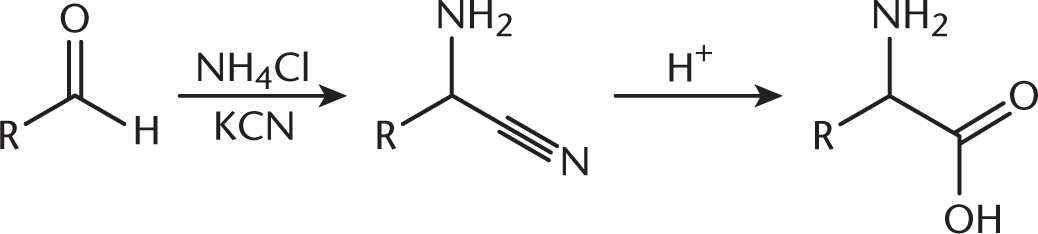
During the Strecker synthesis, ammonia attacks a carbonyl, forming an imine, (B). This imine is attacked by cyanide, forming an amine, (D), and a nitrile, (A). Amide bonds are formed between amino acids but do not appear during the Strecker synthesis. - C
The Strecker synthesis creates an amino acid from an aldehyde. The carbonyl carbon ultimately becomes the α-carbon of the amino acid. Any remaining alkyl chain becomes the R group, as shown below. The starting compound is therefore 2-methylpropanal (isobutyraldehyde).
- D
One resonance structure of a C–N bond in an amide has the double bond between the C and N, not between the C and O. Thus, the C–N bond of an amide has some sp2 character, and sp2-hybridized atoms exhibit planar geometry. - D
Both the Strecker and Gabriel syntheses contain planar intermediates, which can be attacked from either side by a nucleophile. This results in a racemic mixture of enantiomers, and the solution will therefore be optically inactive. - A
During the Gabriel synthesis, phthalimide attacks a secondary carbon in diethyl bromomalonate. The secondary carbon is the electrophile, (D), and bromide is the leaving group, (C). - C
The Gabriel synthesis includes two nucleophilic substitution steps, followed by hydrolysis and decarboxylation. Dehydration—the loss of a water molecule—is not a part of this reaction. - B
The pKa2 of phosphoric acid is close to physiological pH; therefore,  at this pH.
at this pH.
- C
Pyrophosphate is unstable in aqueous solution and will degrade to form two equivalents of inorganic phosphate. The solvent is water, which should retain its polarity regardless of the presence of solutes, eliminating (D). Pyrophosphate and inorganic phosphate are small, charged molecules which are relatively soluble, eliminating (A). - B
The amino acid in question is aspartic acid, which is an acidic amino acid because it contains an extra carboxyl group. At neutral pH, both of the carboxyl groups are ionized, so there are two negative charges on the molecule. Only one of the charges is neutralized by the positive charge on the amino group, so the molecule has an overall negative charge. - A
As DNA is synthesized, it forms phosphodiester bonds, releasing pyrophosphate, PPi. Pyrophosphate is an inorganic phosphate-containing molecule, but it is not the single phosphate group commonly referred to as inorganic phosphate, (B). The DNA molecule itself is referred to as an organic phosphate, (D). - B
Phosphoric acid has three hydrogens with pKa values spread across the pH range. This allows some degree of buffering over almost the entire standard pH range from 0 to 14.