35 Campylobacter species
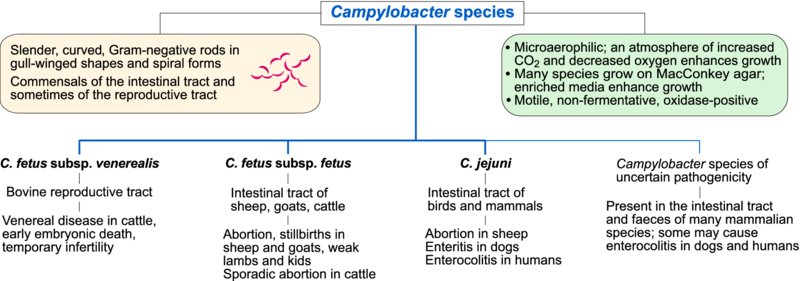
A number of morphological features distinguish Campylobacter species from other Gram-negative bacteria. They are slender, curved, motile, Gram-negative rods with polar flagella. Daughter cells which remain joined have a characteristic gull-winged appearance and long spirals formed by joined cells also occur. These microaerophilic organisms grow best on enriched media in an atmosphere of increased CO2 and decreased oxygen tension. Many Campylobacter species grow on MacConkey agar. They are non-fermentative and oxidase-positive. Campylobacter species are found in the intestinal and genital tracts of domestic animals and are widely distributed geographically. Campylobacter jejuni subsp. jejuni (referred to as C. jejuni) and C. lari colonize the intestines of birds which can result in faecal contamination of watercourses and stored food. Campylobacter fetus subsp. venerealis appears to be adapted principally to bovine preputial mucosa. Campylobacter species are strictly microaerophilic, requiring an atmosphere of 5–10% CO2 for growth. Some species such as C. jejuni grow optimally at 42°C. A selective enriched medium such as Skirrow agar is usually used for primary isolation. Because differentiation of isolates is difficult using conventional cultural and biochemical methods, molecular methods are increasingly used for definitive speciation and for strain typing. PCR-based methods are used for both identification of clinical isolates and for direct detection of Campylobacter species in clinical specimens. Pulsed-field gel electrophoresis is frequently employed for epidemiological investigation of clinical outbreaks of disease in animals and for food-borne infections caused by C. jejuni in humans.
The most important consequences of infections with organisms in this group are infertility in cattle due to C. fetus subsp. venerealis and abortion in ewes caused either by C. fetus subsp. fetus or by C. jejuni. In many developed countries, C. jejuni is the most frequent cause of bacterial food poisoning in humans.
Bovine genital campylobacteriosis
Campylobacter fetus subsp. venerealis, the principal cause of bovine genital campylobacteriosis, is transmitted during coitus to susceptible cows by asymptomatic carrier bulls. The disease is characterized by temporary infertility associated with early embryonic death, return to oestrus at irregular periods and, occasionally, by sporadic abortion. Information on virulence mechanisms is limited but the organism possesses a protein microcapsule or S layer, which confers resistance to serum-mediated destruction and phagocytosis. In addition, a number of different antigenic variants in the S layer may promote the organism's ability to evade the host immune response.
About one-third of infected animals become carriers, with the organisms persisting in the vagina of carrier cows. Extension of infection to the uterus with the development of endometritis and salpingitis can occur during the progestational phase of the oestrus cycle. The infertile period following uterine invasion can last for up to five months, after which specific immunity may develop. This protective immunity may last for up to four years. Campylobacter fetus subsp. fetus, an enteric organism acquired by ingestion, can cause sporadic abortions in cows.
Investigation of the breeding records and vaccination history of an affected herd may suggest campylobacteriosis. Campylobacter species can be detected by the fluorescent antibody technique in sheath washings from bulls or cervicovaginal mucus from cows. Isolation and identification or molecular detection of C. fetus subsp. venerealis from preputial or vaginal mucus is confirmatory. Dihydrostreptomycin, administered either systemically or topically, is used for treating bulls. Intrauterine administration of dihydrostreptomycin can be used therapeutically. Vaccination with bacterins in an oil emulsion adjuvant is used therapeutically and prophylactically in problem herds in some countries.

Ovine genital campylobacteriosis
Campylobacteriosis in ewes may be caused by either C. fetus subsp. fetus or C. jejuni. The disease, which is worldwide in distribution, is one of the most common causes of ovine abortion in some countries. The relative importance of these two species differs in different geographical regions. Since the 1990s, C. jejuni has predominated as a cause of abortion in the USA and a new highly virulent tetracycline-resistant clone has emerged there in recent years. This change in tetracycline resistance may reflect the widespread use of tetracycline for control of ovine abortion in that country. Both Campylobacter species cause abortion in the UK whereas C. fetus subsp. fetus is more prevalent in New Zealand. Campylobacter fetus subsp. fetus is found in the faeces of cattle and sheep and C. jejuni may be present in the faeces of a wide range of birds and mammals. Transmission of both of these organisms is by the faecal–oral route. During pregnancy, localization in the uterus of susceptible ewes may follow bacteraemia. The subsequent necrotic placentitis may result in abortion late in pregnancy, stillborn lambs or weak lambs. Round, necrotic lesions up to 2 cm in diameter with pale raised rims and dark depressed centres are evident on the liver surface in some aborted lambs. Aborting ewes are major sources of infection for susceptible animals in a flock. Up to 20% of ewes in a susceptible flock may abort. Recovered ewes are immune for at least three years.
If present, typical hepatic lesions in aborted lambs are pathognomonic. Isolation and identification of C. fetus subsp. fetus or C. jejuni from foetal abomasal contents or birth fluids is confirmatory. Aborting ewes should be isolated and placentae and aborted foetuses promptly removed. The remainder of the flock should be moved to clean pasture. After confirmation of the disease in a flock, vaccination of ewes with a C. fetus subsp. fetus bacterin is reported to reduce the number of abortions.
Intestinal campylobacteriosis in dogs
Diarrhoea in dogs and other domestic animals has been attributed to infection with Campylobacter species, particularly C. jejuni. Confirmation is difficult because healthy animals may shed Campylobacter species in their faeces. However, the presence of large numbers of campylobacter-like organisms in dilute carbol fuchsin-stained faecal smears or rectal scrapings from dogs with diarrhoea may be indicative of infection. Campylobacter species may contribute to the severity of enteric disease in dogs infected with other enteropathogens such as enteric viruses, Giardia species and helminths. Dogs shedding C. jejuni are a potential source of human infection.
Intestinal campylobacteriosis in humans
Campylobacter jejuni is the main cause of human intestinal campylobacteriosis. Campylobacter coli and C. lari are sometimes implicated. These zoonotic infections are usually food-borne and poultry meat is the main source of human infection. Fever, abdominal pain and diarrhoea, sometimes with blood, are the most common manifestations of this enteric infection. The emergence of antimicrobial resistance in campylobacters, particularly to fluoroquinolones and macrolide antibiotics, is a major public health concern.