48 Pathogenic algae and cyanobacteria
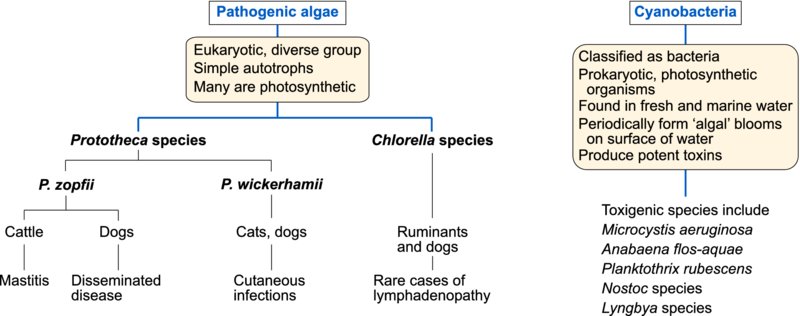
Algae are saprobic eukaryotic organisms which are widely distributed in the environment especially in water. Many contain chlorophyll. Infrequently, some species of algae have been implicated in disease of domestic animals. Colourless eukaryotic algae belonging to the genus Prototheca can invade tissues causing cutaneous and disseminated disease in a number of species and mastitis in cattle. Green algae belonging to Chlorella species have been associated with tissue invasion in ruminants on rare occasions. The prokaryotic cyanobacteria (formerly known as blue-green algae) produce potent toxins which can affect hepatic and neurological function.
Prototheca species
The saprobic colourless algae, Prototheca species, are widely distributed. It is thought that Prototheca species may be achlorophyllous descendents of Chlorella species. Prototheca zopfii has been associated with disseminated protothecosis in dogs and with mastitis in cows. Three biotypes of P. zopfii have been described. Subsequent studies based on sequence analysis of the 18S rRNA gene have led to the proposal that P. zopfii should be reclassified as genotypes 1 (biotype 1) and 2 (biotype 2), and a new species P. blaschkeae (biotype 3). The majority of bovine protothecal mastitis cases are caused by P. zopfii genotype 2. Cutaneous protothecosis in cats and dogs is caused by P. wickerhamii. Prototheca species grow aerobically forming yeast-like colonies on Sabouraud dextrose agar and on blood agar. During asexual reproduction two to sixteen sporangiospores develop within a sporangium.
Infections due to Prototheca species are opportunistic and infrequent. Organisms can enter tissues at sites of minor trauma in skin and mucous membranes or through the teat canal. Some outbreaks in cattle have been associated with the use of contaminated intramammary products. A cutaneous form of protothecosis, caused by P. wickerhamii, is the only manifestation of the disease reported in cats characterized by large, firm, discrete nodules on limbs and feet. Infection of dogs with P. zopfii probably occurs through the intestinal mucosa as dissemination is often preceded by haemorrhagic colitis. Affected dogs present with protracted bloody diarrhoea along with signs of neurological or ocular disturbance. There may be progressive weight loss and debility. Prototheca zopfii can cause chronic progressive pyogranulomatous lesions in bovine mammary glands and associated lymph nodes. Indurative mastitis may affect a number of quarters. Prototheca zopfii can persist in the tissues throughout a dry period and may be excreted during the next lactation. Treatment of protothecosis is difficult and may ultimately be unsuccessful.
Suitable specimens for laboratory examination include milk samples and biopsy or postmortem tissues. An indirect ELISA has been described for the detection of antibodies in serum and whey. Methenamine silver or PAS techniques can be used to demonstrate algal cells and sporangia in histological sections of granulomatous lesions. Immunofluorescent techniques are used to identify P. zopfii and P. wickerhamii in tissues. The organisms grow on blood agar and Sabouraud dextrose agar without cycloheximide. Culture plates are incubated aerobically at 35–37°C for two to five days.
Chlorella species
Green algae cause disease in ruminants on rare occasions. Chlorella species are morphologically similar to Prototheca species. However, they are photosynthetic, possessing chloroplasts containing green pigment which imparts colour to infected tissues. In Australia, the organisms have been recovered from liver and associated lymph nodes of sheep and from cattle with lymphadenitis. Disseminated chlorellosis has been described in a dog.
The cyanobacteria
These prokaryotic photosynthetic organisms are found worldwide in fresh and marine water and in soil. Blue-green ‘algal’ blooms may form when conditions allow rapid replication of cyanobacteria. They occur in water, enriched with phosphates or nitrogen, when its temperature is between 15 and 30°C, its pH is neutral or alkaline, and wind disturbance is minimal. Domestic or wild animals drinking contaminated water are likely to be exposed to toxin released from the organisms. More than 40 species of cyanobacteria are known to produce potent hepatotoxins or neurotoxins. Microcystis aeruginosa is hepatotoxic and the species most often incriminated in episodes of poisoning. Some species such as Anabaena flos-aquae can generate both hepatotoxin and neurotoxin.
Toxins of the cyanobacteria, their modes of action and their clinical effects are presented in Table 48.1. Although death may occur within a short time after ingestion of a lethal dose of toxin, the dose–response curve is relatively steep and animals can ingest nearly 90% of a lethal dose without noticeable effects. Birds and ruminants are usually more susceptible to the toxins than monogastric animals.
Table 48.1 Toxins of cyanobacteria, their modes of action and clinical effects.
| Toxins | Mode of action | Clinical effects |
| Microcystins and nodularins | Hepatotoxic; inhibition of protein phosphatases | Hepatomegaly and hepatoencephalopathy; photosensitization; raised serum liver enzyme levels; severe toxicity results in intrahepatic haemorrhage and death from hypovolaemic shock |
| Anatoxin-a | Neurotoxic; postsynaptic cholinergic agonist; mimics the activity of acetylcholine | Involuntary muscular contractions, convulsions; severe toxicity results in death |
| Anatoxin-a(s) | Neurotoxic; anti-acetylcholinesterase activity | Similar to the effects of anatoxin-a; hypersalivation |
| Saxitoxins and neosaxitoxins | Blockade of signal transmission in motor neurons | Flaccid paralysis; death from respiratory failure |
Affected animals may have a history of access to contaminated water with an ‘algal’ bloom and their mouths or legs may be stained green. Samples of bloom should be examined microscopically for the presence of cyanobacteria. Toxin must be demonstrated in the bloom or in stomach contents by chemical, biological or immunoassay techniques in a reference laboratory.
Affected horses and ruminants should be removed from the source of toxin and housed out of direct sunlight. Emetics administered to recently exposed dogs may aid recovery. Activated charcoal slurry or ion-exchange resins may be used for adsorbing toxins from the gastrointestinal tract. Animal access to contaminated water must be restricted. The treatment of an ‘algal’ bloom with algicides results in the liberation of toxins from dead cells into the water.