2. A single hair
Take a firm hold of one of the hairs on your head and pull it out. No one said science was going to be entirely painless. If you want to make this less stressful, get a hair from a hairbrush. If you are bald, get hold of someone else’s hair – but ask first! Now, examine what you’ve got. It’s a long, very narrow cylinder, flexible yet surprisingly strong considering how thin it is.
Take as close a look at the hair as you can. If you can lay your hands on a microscope, use that, but otherwise use a magnifying glass.
That strand of hair is going to start us off on everything from philosophy to physics. Dubious about just how philosophical hair can be? Consider this: you are alive and that hair is an integral part of you (or at least it was until you pulled it out). Yet the hairs on your body are dead – they are not made up of living cells. The same is true of fingernails and toenails. So you are alive, but part of what goes to make ‘you’ is dead.
Remember that next time a TV advert is encouraging you to ‘nourish’ your hair. You can’t feed hair. You can’t make it healthy. It’s dead. Deceased. It has fallen off its metaphorical perch. Worried that your hair is lifeless? Well, don’t be. That’s how it is supposed to be. It’s quite amazing just how many hair products are advertised using the inherently meaningless concept of ‘nourishing’.
We’re talking about a single hair, but of course you have (probably) got many more than one on your head. A typical human head houses around 100,000 hairs, though those with blonde hair will usually have above the average, and those with red hair rather fewer. Looking at that individual hair, the colour that provides this distinction doesn’t stand out the same way it does on a full head of hair, but it’s still there.
The colours of nature
The colour in hair comes from two variants of a pigment called melanin. One, pheomelanin, produces red colours. Blonde and brown hair colourings are due to the presence of more or less of the other variant of the pigment, eumelanin. This is the original form of hair pigment – red hair is the result of a mutation at some point in the history of human development.
As we become older, the amount of pigment in our hair decreases, eventually disappearing altogether. Grey and white hairs don’t have any melanin-based pigment inside. In effect they are colourless, but the shape of the hair and its inner structure has an effect on the way that the light passes through it, producing grey and white tones.
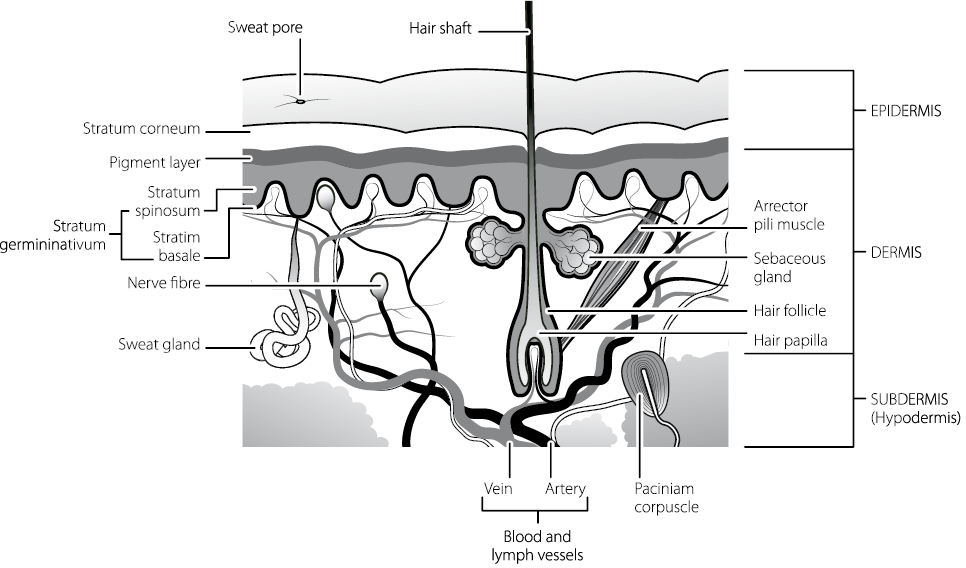
The inner structure of hair isn’t particularly obvious when you hold a single strand in your hand and look at it with the naked eye, but under a microscope it becomes clear that there is more going on than just a simple filament of uniform material. In effect your hairs have three layers: an inner one that is mostly empty, a middle one (the cortex) that has a complex structure that holds the pigments and can take in water to swell up, and an outer layer called the cuticle which looks scaly under considerable magnification, and which has a water-resistant skin.
On the end of the hair, where you have pulled it out of your scalp, there may be parts of the follicle, the section of the hair usually buried under your skin. The follicle is responsible for producing the rest of the structure and is the only part of the hair that is alive.
Dyeing to be attractive
The idea that the colouring of your hair is produced by melanins assumes it has its natural hue, but many of us have changed our hair colour using dyes at one time or another. Dyes use a surprisingly complex mechanism to carry out the superficially simple task of changing a colour. It’s not like slapping on a coat of paint – the process of dyeing hair owes more to the chemist’s lab than the beauty salon.
In a typical permanent dyeing process, a substance like ammonia is used to open up the hair shaft to gain access to the cortex. Then a bleach, which is essentially a mechanism for adding oxygen, is used to take out the natural colour. Any new colouration is then added to bond onto the exposed cortex. Temporary dyes never get past the cuticle; they sit on the outside of the hair and so are easily washed off.
Worrying about hair loss
Almost every human being has hairs, but compared with most mammals we are very scantily provided. Not strictly in number – we have roughly the same number of hairs as an equivalent-sized chimpanzee – but the vast majority of these hairs are so small as to be practically useless.
Next time you are cold or get a sudden sense of fear, take a look at the skin on your arms. You should be able to see goose bumps or goose pimples. This hair-related (indeed, hair-raising) phenomenon links to the fact that our ancestors once were covered in a thick coat of fur like most other mammals.
When you get goose bumps, tiny muscles around the base of each hair tense, pulling the hair more erect. If you had a decent covering of fur this would fluff up your coat, getting more air into it, and making it a better insulator. That’s a good thing when you are cold, at least if you have fur – now that we’ve lost most of our body hair, it just makes your skin look strange without any warming benefits.
Similarly, we get the bristling feeling of our hair standing on end when we’re scared. Once more it’s a now-useless ancient reaction. Many mammals fluff up their fur when threatened to make themselves look bigger and so more dangerous. (Take a dog near to a cat to see the feline version of this effect in all its glory. The cat will also arch its back to try to look even bigger.) Apparently we used to perform a similar defensive fluffing-up, but once again the effect is now ruined by our relatively hairlessness. We still feel the sensation of having our hair stand on end, but get no benefit in added bulk.
Our lack of natural hairy protection struck me painfully when out walking my dog recently. It was a cold day and I was under-dressed for the weather in a short sleeved shirt. I was shivering and my trainers were soaked from the wet grass, so that I squelched as I walked. When passing through the fence from one field to the next, I managed to brush against a rampant clump of nettles, stinging both my arms.
But the dog, with her thick fur coat and hard padded feet, was impervious to both the weather and the vegetation. She seemed much better prepared to survive what nature could throw at her than I was.
I wondered why human beings are so badly equipped to cope with the discomforts and dangers of the natural world. We know that our distant ancestors had good, thick coats of protective fur, just as the apes still do today. (Present-day apes like chimpanzees and gorillas aren’t our ancestors, but it’s a mistake that’s still often made in describing them.) It seems counter-intuitive that the early humans should have lost that helpful fur.
Of course, it’s a misunderstanding to think that evolution has our best interests in mind. Evolution doesn’t have a mind, or any concept of what is good or bad for us. Evolution usually works by gradual selection of subtle variants that enhance the survival and reproduction capabilities of individual members of species. It doesn’t take an overview and think ‘That’s good, I’ll keep that’. Even so, it seemed unlikely that there was any evolutionary benefit in losing the warmth and protection of that natural fur coat.
Just because evolution deals us a set of cards it doesn’t mean that everything we receive in our genetic hand is beneficial. There doesn’t have to be an obvious evolutionary advantage just because we have developed a certain trait. It’s just as likely to be a side effect of another evolutionary development. For example, many birds have wings that are easily snapped, because the bones are thin and hollow. Having weak bones isn’t a good thing in itself – on the contrary, it’s bad for survival. However, it is necessary to reduce the bird’s weight enough for it to be able to fly.
There are various possibilities as to why it made evolutionary sense to lose the majority of our hair. It might have been due to the need to sweat more as our ancestors moved from the forest to the savannah – it’s easier to sweat with less hair, exposing more skin for sweat to evaporate. Equally it could have been a response to the increase in parasites (though all the great apes are afflicted with these). Most exotically it has been suggested that early humans were partly aquatic, and less body hair made for a sleeker swimmer (though many semi-aquatic mammals are hairy). But the explanation that works best for me is that the loss was an accidental side effect, like those precariously thin bird bones.
To make allies, lose your hair
Around 100,000 years ago our distant ancestors went through the final changes that made them into modern humans. That was the end of our evolution to date. We are the same biological species now as they were back then. There have been plenty of tiny changes at the genetic level, but as a species we are essentially the same. We have the same potential for physical strength, for longevity, for attracting the opposite sex, for thinking and more.
Those many thousands of years ago, our predecessors had undergone huge evolutionary changes from the common ancestor they shared with chimpanzees and the other great apes. The pre-humans had lost most of their hair, leaving a delicate, thin skin exposed. They had shifted from a four-legged gait to walking upright. Their brains had grown out of all proportion with their bodies, leaving them bulgy-headed and top heavy (quite possibly unattractive features at the time). Their mouths had become smaller, making their teeth less effective as a biting weapon. The big toe had ceased to be an opposing digit that could be used to grip a tree branch.
Taken together, these alterations made the pre-humans more vulnerable to attack by predators. Their naked, unprotected skin was pathetically easy for claws and teeth to rip through. Compared with the smooth, four-footed pace of other apes, their tottering movements on two legs were painfully clumsy – a rabbit could easily outrun this strange unstable creature. The adaptations that came through in pre-humans don’t seem to make any sense except as side effects. Put them alongside the change of behaviour that may have triggered them, and they were an acceptable price to pay.
These physical modifications of pre-humans are likely to have been an indirect result of an environmental upheaval. As the global climate underwent violent change, our ancestors were pushed out of the protective forests into the exposed world of the savannah. Facing up to starkly efficient predators, they were forced to change behaviour or become extinct. Back then, most pre-humans could not function well in large groups. This is still the case with most of our close relatives. The chimpanzee, for example, is incapable of forming large, cooperative bands. Get more than a handful of males together and the outcome is bloody carnage as battles for supremacy break out.
The pre-humans who first straggled onto the savannah around five million years ago were probably much the same. But the fast, killing-machine predators of the day – from the terrifying sabre-toothed dinofelis and the lion-sized machairodus to the more familiar hyena – made sure that things changed. The most likely pre-humans to survive were those with a natural tendency to cooperate. Our ancestors began to live in larger groups, giving them the ability to take on a predator and win, where a small band would be torn to pieces. And this change of behaviour may well have brought with it as side effects all the physical oddities that we observe in modern man.
The characteristics that repressed aggression and enhanced the ability to cooperate are typical of juvenile apes. Our primate cousins’ inability to function in large groups only appears with maturity. The individuals amongst our predecessors who were more likely to survive on the savannah, those with the immature ability to get on with their fellows rather than tear them to pieces, were also the least physically developed. The eventual outcome was lack of hair on most of the body, a large head, a small mouth and even the upright stance – all features of the early part of the primate lifecycle that have normally disappeared by the time an individual matures.
As an aside, this mechanism of selecting for cooperative behaviour and getting an infant-like version of the animal is something humanity has since managed to produce repeatedly in its domestic animals. The dog, for example, has much more in common with a wolf cub than with the mature wolf that it was bred from. This is not just a matter of theory. In a fascinating long-term experiment between the 1950s and the 1990s, Russian geneticist Dmitri Belyaev selectively bred Russian silver foxes for docile behaviour and showed just how early man managed to turn the wolf into a dog.
Over 40 years – an immensely long experiment, but no time in evolutionary terms – the fox descendants began to resemble domesticated dogs. Their faces changed shape, becoming more rounded. Their ears no longer stood upright, but drooped down. Their tails became more floppy. Their coats ceased to be uniform in appearance, developing colour variations and patterns. They spent more time playing, and constantly looked for leadership from an adult. As they became more cooperative, they took on the physical appearance and the behaviour patterns of overgrown fox cubs.
To get back to humans, in the process of becoming more cooperative, and so more infantile (neotenous in the scientific jargon), the pre-humans lost the majority of their hair, leaving us with the largely hairless appearance we have today. Except, of course, on our heads. Head hair can be lush in the extreme, and unlike the rest of our body hair (and that of other mammals) it just keeps on growing.
As with our general lack of hair, there are several possible explanations for this. It’s quite possible that originally all our hair stayed at a roughly fixed length, but over time natural selection moved us towards head hair that continued to grow. This could be because those with a mutation causing head hair to keep growing had better protected brains. Or it could have been a side effect of wearing clothes, leaving the head most in need of furry protection. Or it could have provided a shield against the full impact of the noonday Sun, which can be formidable (as anyone with a bald patch can testify). Or there might be another, quite different explanation.
Tracing back the ‘reason’ for an evolutionary trait like this is notoriously difficult because we can’t directly observe what happened or do an experiment to test a particular theory. It’s a bit like news analysis saying that the stock market fell ‘because of lack of confidence in the government’, or for some other reason. No one really knows for certain why the market reacted this way, and similarly no one can prove why humans developed a particular trait. It is inevitably a matter of conjecture.
Lost in space
But given that we are now largely hairless, in some circumstances, clothing is a survival essential. Whether you are venturing under the sea or to the North Pole, your clothing is part of your equipment. And perhaps the greatest example of clothes-as-protection is when someone is out in space. Your body was never intended to be exposed to the extremes of space. The temperature is impossibly cold, as low as –270°C. There is no atmosphere. It’s literally like nothing on Earth. Yet astronauts regularly make spacewalks protected only by specialist clothing.
It is possible to survive in space briefly without the right protection. Hollywood loves showing what would happen to a human being exposed unprotected, and can get it wonderfully wrong. The most ludicrous example is in the 1990 Arnold Schwarzenegger movie Total Recall, based on a Philip K. Dick story, where, expelled from the protected environment of a city on Mars, human beings inflate grossly before their heads explode messily.
Mars actually has a slight atmosphere (around one per cent of Earth’s atmospheric pressure), and even in space this sort of inflation and explosion caused by low pressure isn’t going to happen. There would be some discomfort as gas escaped from body cavities, but there is no danger that your head would inflate like a balloon.
It is true, though, that you would experience some liquids boiling. The lower the pressure, the lower the boiling point of anything, and in space – with no pressure to speak of – you will get an unpleasant drying up of the eyes as water boils away. Some fiction assumes your blood will boil in your veins, too – a horrible way to go – but according to NASA the pressure of your skin and circulatory system is enough to stop this happening.
Another worry is that you would freeze instantly in the very low temperatures of space. But bear in mind how a vacuum flask keeps its contents piping hot. Heat can only travel through a vacuum as light. We get our heat from the Sun in the form of light, which can happily cross empty space. Admittedly our bodies do glow with infrared – they do give off a degree of (invisible) light. But most of the heat we usually lose is passed on by conduction. The heat in our skin – atoms jiggling around with thermal energy – is passed on to the atmosphere, so our atoms jiggle a bit less, and the atmospheric atoms jiggle a bit more. That can’t happen in a vacuum.
You would lose heat, but not very quickly. In practice, the thing that is going to kill you in space is simply the lack of air to breathe, and this will take a number of seconds. NASA has even experienced what would happen, when in 1965 a test subject’s suit sprang a leak in a vacuum chamber. The victim (who survived) stayed conscious for around fourteen seconds in the airless chamber. According to NASA, the exact survival limit isn’t known, but would probably be one to two minutes.
There’s no doubt, then, that clothes can be important survival aids. Yet most of us, in everyday life, only have to cope with environments where plenty of other animals manage perfectly well with a bit of fur and some hardened skin on the feet. As naturists demonstrate, wearing clothes is often a social decision rather than an essential protection, and it’s a decision we’ve been making for a long time. Woven cloth dates back at least 27,000 years – we know this because clay has been found at an ancient settlement at Pavlov in the Czech Republic with the imprint of woven cloth on its surface.
This isn’t the oldest evidence for clothes we have, though. Bone needles have been found at Kostenki, a village in Russia, dating back around 40,000 years. These seem to have been used to stitch together animal skins to provide clothing. But the best clues to just how long we have been wearing clothes comes from the humble louse.
A lousy measurement
When Robert Hooke published Micrographia (see page 51), probably the most delight and revulsion came from his fold-out illustration of a louse. Seen magnified they are truly evil-looking parasites, specialist bloodsuckers that live on their host’s skin, taking sips from the blood beneath. As many people with children at junior school know, the head louse is very fussy about sticking with its preferred environment around the base of head hairs. You don’t find head lice straying to other parts of the body. But it does have a cousin that’s less picky.
The human body louse evolved from the head louse between 50,000 and 100,000 years ago. We don’t have ancient lice to work this out from, but this timing can be estimated by looking at the variations in the DNA of the two creatures – the more difference, the longer ago the division between head and body lice occurred.
This is of interest when thinking about the history of clothing because it’s thought that the body louse was only able to develop once we started wearing clothes. Before then, the uncovered skin was too exposed. Interestingly, this 50,000 to 100,000 year timescale corresponds well with the timing of the move of humans out of Africa into colder climates, which could have been the spur that brought on the use of clothing.
Getting under your skin
Underneath your clothes, your body is covered in skin. Like hair, skin relies on melanin-based pigments to get its colouring. Also like hair, the outer layer of your skin is dead. The tiny flakes that contribute to the dust around your house fall off from this surface. Immediately below that dead layer called the stratum corneum (like the cornea in the eye, this ‘corneum’ comes from the Latin for horn, cornu) are two further layers, protective squamous cells and basal cells. The basal cells rise to the surface where they die, to form the outer coating, and they also play host to a different kind of cell, melanocytes, which produce skin pigments.
The more melanin the melanocytes pump out, the darker your skin. The normal state of your skin will have evolved to match the amount of ultraviolet in the light where your ancestors lived. Ultraviolet sits on the spectrum of light between visible light and x-rays – it is energetic enough to cause damage to the DNA inside your cells, if it can penetrate the outer layers of skin. Humans with a history of low exposure to ultraviolet – in the northern hemisphere – tend to lose melanin from the original African levels of their common ancestors.
This reduction in protection might not seem to have any advantage, merely adding risk if you get exposed to more sunlight (for example by emigrating to Australia), but in practice it was beneficial. This is because, despite the risk, the body needs some ultraviolet to get through, as it is used to produce the essential vitamin D. This is a vitamin that is relatively uncommon in food and that we need to avoid conditions like rickets. In northern climates, where there isn’t as much sunlight, the early settlers needed more ultraviolet to be allowed through.
This led to paler skin in northern areas, and what melanin the northerners were left with can often clump together to make dark patches, forming freckles and moles. Even in areas where sunlight tends to be weak, levels of ultraviolet can vary, so the skin has a mechanism – tanning – to deal with varying strength of UV. When the skin is exposed to strong sunlight, the melanocytes go into overdrive, producing more melanin and darkening the skin, thereby allowing it to absorb more ultraviolet and preventing damage to the lower layers.
What is stuff made of?
Keratin, the main structural material of the outer layers of both your skin and your hair, is a protein. And a protein is a molecule, a collection of atoms. If you go back to the hair you pulled from your head and start to zoom in, taking in more and more detail, you will eventually get down to the fundamental building blocks of the universe. To understand how your body is constructed, we have to ask what is ‘stuff’ (including your hair) made of?
The Ancient Greeks had two theories. The dominant idea was that everything was made up from four ‘elements’ – earth, air, fire and water. However, a small but vocal opposition thought that if you took stuff and cut it into smaller and smaller pieces you would eventually get to the limit of that cutting. The remaining piece would be uncuttable or a-tomos: they thought everything was made up of atoms. This idea stayed on the back burner for almost 2,000 years, until in the early 1800s, English scientist John Dalton devised modern atomic theory, suggesting that the different elements were made up of different types of small particle called atoms, each type unique to an element.
These elements were not the Ancient Greek four, but chemicals that could not be made out of others. Gases like hydrogen and oxygen, metals like iron and lead, and other substances like carbon and sulfur (for UK readers who think this word looks odd, this is now the standard worldwide chemical spelling for sulphur). Yet even at the start of the twentieth century, most scientists believed that atoms were just a useful concept to make chemistry work, rather than actual entities. It was only with work started by Albert Einstein in 1905 that atoms were finally considered to be real.
Battered by molecules
Atoms are a bit like small children – they are never entirely still. If you look at a glass of water sitting on a table, the water seems motionless. Yet within it, the water molecules are frantically (if randomly) rushing around. Einstein realised that an effect first observed by Scottish botanist Robert Brown in 1827 could be explained by the clumsiness of these energetic molecules.
Brown had spotted that the pollen grains of an evening primrose plant danced around in a drop of water when watched under a microscope. At first, Brown thought this was because there was some kind of life force in the pollen, but the same thing happened with ancient pollen and with stone dust and soot. It wasn’t life in the pollen, but the activity of the water itself that created this ‘Brownian motion’. Einstein realised that it was the water molecules randomly bashing into the pollen grains that caused the movement, and went on to give a mathematical basis for the theory. A little later, in 1912, French physicist Jean Perrin performed a wide range of experiments proving for the first time that atoms and molecules exist.
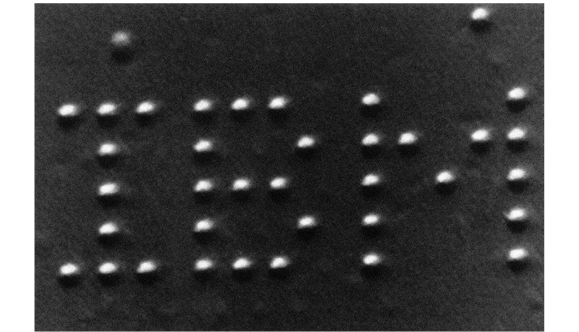
Remarkably, individual atoms can now both be manipulated and experienced visually. In 1989 a team working at IBM was the first to use a type of electron microscope that can manipulate as well as view, in order to move an individual atom. Two months later they arranged 35 atoms of the element xenon to spell out the initials IBM.
A little earlier, in 1980 Hans Dehmelt of the University of Washington isolated a single barium ion (an ion is just an atom with electrons missing, or extra electrons added, giving it an electrical charge). When illuminated by the right colour of laser light, that individual barium ion was visible to the naked eye as a pinprick of brilliance floating in space. You might argue that you couldn’t ‘see’ the ion, just light that was reflected by it – but then that’s all that ever happens when we see something.
Empty atoms and electromagnetic bottoms
The atoms that make up your body are not only very small, they are also mostly composed of empty space. If you could squeeze all the matter in your body together, removing the gaps, it would pack into a cube less than 1/500th of a centimetre on each side.
One of the wonders of the cosmos is the neutron star, a star in which the atoms have collapsed, losing all that empty space. In a single cubic centimetre of neutron star material – a chunk little more than the size of a sugar cube – there are around 100 million tons of matter. The entire star, heavier than our Sun, occupies a sphere that is roughly the size across of the island of Manhattan.
There is no danger of the atoms in you or your hair collapsing like a neutron star – without the massive gravitational pull of the star they remain stable. Collections of such atoms make up molecules like the keratin in your hair. The atoms stay together because of electromagnetism, one of the four forces of nature we will meet in more detail in Chapter 6. A molecule can be made up of a single element, like oxygen, the gas we breathe, which comes in molecules of paired atoms. Or it can be a compound, linking different elements, anything from simple sodium chloride – common salt – to the complex molecules, found in living organisms, like keratin.
The atoms that everything is composed from never touch each other. The closer together they get, the greater the repulsion between the electrical charges on their component parts. It’s like trying to bring like poles of two intensely powerful magnets together. This is even the case when something appears to be in contact with something else. When you sit on a chair, you don’t actually touch it. Your body floats an infinitesimal distance above, suspended by the repulsion between atoms.
It may be quite a while since you’ve played around with magnets. Get hold of a couple and remind yourself how remarkable the interaction between them really is.
Somehow the repulsion when you bring two of the same pole together seems more magical than attraction. Yet this is exactly what is happening every time one piece of matter ‘comes into contact’ with another. The interaction is electrical rather than magnetic, but it’s a similar electromagnetic repulsion to the one you feel between the magnets that stops the atoms in your bottom slipping between the atoms of the chair.
Exploring an atom’s innards
It wasn’t long after atoms were proved to exist in 1912 that it turned out that the name was inaccurate. Atoms aren’t ‘uncuttable’. They have component parts. Scientists were already aware that there were negatively charged particles called electrons that could be pulled out of atoms. At first these were assumed to be scattered through a mass of positive material, like plums in a plum pudding (a description provided by British physicist J.J. Thomson). But a walrus-moustached New Zealander working in Cambridge proved things were different.
Ernest Rutherford had the idea of firing other particles into an atom and seeing how they reacted – a bit like throwing a ball at an invisible structure and using the way the ball is influenced by what it hits to work out what that structure is like. The ‘ball’ he used was an alpha particle, a particle that had recently been discovered shooting out of radioactive elements. (It was later identified as the nucleus of a helium atom.) Alpha particles made tiny flashes when they hit screens painted with fluorescent material. By crouching in the dark it was possible for Rutherford’s assistants to spot the paths of particles that were deflected to the sides as they were shot at a piece of gold foil.
With the kind of inspiration that makes all the difference in science, Rutherford and his team also looked for alpha particles that reflected off the atoms in the gold straight back towards the source – and occasionally one did. This was totally unexpected. Rutherford said it was like firing an artillery shell at a piece of tissue paper and having it bounce back at you. He realised it meant that atoms must have a small, very dense, positively charged core to repel the positive alpha particles. Rutherford established for the first time the familiar picture of an atom being like a solar system with a positive nucleus at the centre (he borrowed the word ‘nucleus’ from biology). The nucleus was the equivalent of the Sun and the negatively charged electrons were the planets of this tiny solar system.
Thomson’s plum pudding was no more. The nucleus was so much smaller than the whole atom it was described as being like a fly in a cathedral, around 100,000 times smaller than the atom as a whole. The nucleus was made up of positively charged particles called protons, making up 99.9 per cent of the mass of the atom. For each proton an electron flew around the outside, balancing up the electrical charge, leaving the atom neutral.
But even this newly detailed picture wasn’t quite good enough. In 1932 another particle was found in the nucleus – the neutron. This had a similar mass to the proton but no charge, and it helped explain a mystery. There exist different versions of the same element, called isotopes. They act in the same way chemically, but the atoms have different weights. The neutron explained this picture. The number of charged particles decide what element you have and how it reacts chemically. But different atoms of the same element can have varying numbers of neutrons in the nucleus, producing a range of weights.
No miniature solar system
When we imagine the atoms making up our bodies, this is the picture of what an atom ‘really’ is that many of us still have, but science has moved on since 1932. We now know that electrons don’t fly around the nucleus like planets around the Sun – the solar system model just doesn’t work. If it was an accurate picture, we’d have problems. When a charged particle is accelerated it gives off energy in the form of light. And orbiting is a form of acceleration. This is because acceleration doesn’t mean a change of speed, which is the sense in which we tend to use the word, but rather a change of velocity.
Speed is just a number, like, say, 30 miles per hour. But velocity is more. It is speed and direction. So it might be 30 miles an hour, due north. Anything moving accelerates if any part of its velocity changes. So even if it is still going 30 miles per hour, it accelerates if it changes from heading north to heading east. If you think about an electron whizzing around in an atom like a miniature planet, it would always be changing direction, always accelerating. And that means it would lose energy as a burst of light and would plunge into the nucleus in a tiny fraction of a second. Every atom in the universe would instantly self-destruct.
Taking a quantum leap
The reason everything doesn’t disappear in a flash is explained by quantum theory, the science of the very small. This tells us that the familiar picture of electrons as little particles, whizzing around in an orbit, is wrong. At any point in time, an electron isn’t in a single position. Instead it is in many places around the atom simultaneously, each with different probabilities, only settling to a single location if it is observed. It’s better to think of them as fuzzy clouds of probability around the outside of the atom. Of course, it’s harder to draw a picture of that, so the old solar system model still features in many textbooks.
The electrons that produce this ‘fuzz’ on the outside of atoms can only exist with specific levels of energy. It’s as if they run on rails. You can give them a boost of energy, in which case they will jump up to the next rail. But you can’t give them an intermediate amount of energy; they can never end up positioned between rails. These fixed ‘packets’ of energy are called quanta, which is where the name ‘quantum theory’ comes from.
This also means that the term ‘quantum leap’ is used very strangely in everyday language. A quantum leap is the jump between one rail and the next one. It’s the smallest possible change in the energy of an electron that there can be. So it is rather bizarre that in general usage it has come to mean a really significant transformation.
Usually, the energy to push an electron to a higher level (the ‘rail’ analogy is mine, it’s not in general usage) is provided by light. Light carries energy (it’s just as well that it does, because that’s how the Sun’s energy reaches us across the vacuum of space) and gives electrons those necessary boosts. Similarly when an electron drops down a level, it gives off light. But because the electron can only move from rail to rail, this energy is in packets – quanta. The light comes in packets – particles – which are called photons.
The charm of quarks
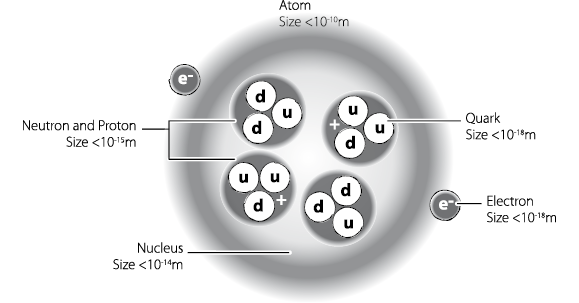
Your body is made of molecules, each containing atoms, each of which has an internal structure of protons, neutrons and electrons. But we know now that the old picture of protons and neutrons being the fundamental objects at the heart of an atom is also wrong. Protons and neutrons are both made of truly fundamental particles called quarks. There are quite a few types of quark, described by their ‘flavour’ (no, really). The different flavours include charm, strangeness, top and bottom, but the ones we’re interested in are up and down. A proton is made of two up quarks and one down, while a neutron is two down quarks and one up.
This all works out in terms of electrical charge, because up quarks have a 2/3 charge and down quarks have –1/3, resulting in a positive charge of 1 for the proton and no overall charge for the neutron. It sounds wrong that a particle should have a fraction of a charge, and quarks aren’t really 1/3 or 2/3 of anything – they are the true units of charge. However, because protons and electrons are all that were known when the numbers were first established, we’re stuck with thirds.
This odd name, quark, is often pronounced to rhyme with lark, but when American physicist Murray Gell-Mann dreamed up the idea, he wanted it to rhyme with cork. He came up with that ‘kwork’ sound without thinking about how to spell it. But then he came across a line in James Joyce’s Finnegans Wake: ‘Three quarks for Muster Mark!’ The way quarks come in threes made the text very apt, so Gell-Mann adopted the spelling, even though it didn’t fit his pronunciation.
The messy standard model
With quarks you have really reached the uncuttable – part of a bigger picture scientists use to describe all the particles that make up your body and the rest of the universe.
Physicists have produced something called the ‘standard model’, which describes everything we know in existence being based on around nineteen different fundamental particles. Twelve of these are matter particles, like quarks and electrons, plus some more obscure variants found in nuclear reactions and collider experiments. Another five are special particles that carry forces. So, for instance, there’s the photon which is both a particle of light and carries electromagnetic force from place to place.
There are also a couple of particles that may or may not exist – the graviton, which would be the particle that carried gravity, if gravity is indeed a force that comes in quantum chunks like the others (as yet this isn’t fully supported by theory). And then there’s the Higgs boson, the main target of the massive Large Hadron Collider at CERN, which is an elusive particle that is thought to give some of the other particles their mass.
To make things even more complex, each particle has an anti-particle. Antimatter sounds like something out of Star Trek (and in fact it is how the Enterprise’s engines are supposed to work), but it’s very real. Antimatter is just like ordinary matter, but some of its properties, like the charge, are reversed. All twelve matter particles have an antimatter equivalent. So, for instance, the electron has the anti-electron, better known as a positron, which has a positive charge instead of a negative one.
If matter and antimatter are brought together they destroy each other and their mass is converted into energy. Because the energy in matter is quantified by Einstein’s famous equation E=mc2, and c, which is the speed of light, is a very big number, there’s a whole lot of energy going on when matter and antimatter combine. A kilogram of antimatter, annihilating with an equivalent amount of matter, generates the equivalent of a typical power station running for around twelve years. (Depending on the antimatter used, there may be secondary particles called neutrinos produced in the reaction, which can reduce the energy output by half, but this is a relatively small consideration.) Antimatter is the most compact way to store energy that we have. It packs in 1,000 times more energy than nuclear fuel.
Although this zoo of different particles works pretty well at explaining everything that goes into the matter than makes up your hair – and everything else with mass or energy – it is a messy way of looking at things, and scientists would love to have a simpler picture to deal with the fundamentals of reality. For years physicists have been developing competing theories to achieve this, but as yet none is satisfactory.
Is it solid, liquid or gas?
Away from such theoretical considerations, an interesting question to ask when looking at your hair is what kind of material it’s made of. You were probably taught at school that all matter is solid, liquid or gas. As a hair clearly isn’t liquid or gas it must be a solid, but something so flexible and pliant doesn’t really fit with our immediate concept of a solid. We tend to think of a solid as rigid, not pliable. Sand is another good example of a substance that doesn’t fit comfortably with simplistic classifications. Think of a fistful of sand – indubitably sand is made of solid particles, yet it runs through your fingers like a liquid.
We can get a better feel for these ‘states of matter’ from one of the few substances that we experience as solid, liquid and gas – water. From it we learn that the distinction between the three states of matter is twofold. The atoms are typically further away from each other and they are typically moving faster as we go from solid to liquid to gas. All atoms and molecules move, but in a solid they jiggle about in a well-established framework of bonds – electromagnetic links between molecules. In a liquid, there are still bonds, but they are less substantial and have no stable structure. In a gas the molecules act pretty well independently.
This makes it sound as if there is a continuum between states, but they are clearly defined. It’s true that as a liquid, for example, molecules of water will constantly be escaping into gaseous form (evaporating), but if you want to turn a body of water into gas you have to heat it to the right temperature, the boiling point, and then give it extra heat (the ‘latent heat of boiling’) to remove the final bonds and let those molecules free.
The fourth state of matter
The science you were taught at school probably stopped with the Victorian idea of there being three states of matter, but in fact there are five states altogether. The fourth is one that you have experienced many times – it is a much more obvious state than gas – but because our school science is so strongly locked into the nineteenth-century worldview, even many adults don’t know it exists, except as a label in relation to large screen TVs. It’s plasma.
One potential point of confusion needs clearing up here, especially as our starting point in this book is your body. This plasma we are discussing has nothing to do with blood plasma. Blood plasma is the colourless liquid in which blood cells float. Plasma in the physics sense is the fourth state of matter, the one that comes beyond a gas. (Actually neither of the uses of the word are particularly good, as ‘plasma’ originally meant something formed or moulded, and both types of plasma are formless.)
It shows how badly plasma is understood that my dictionary defines it as being ‘a gas in which there are ions rather than atoms or molecules’. Let’s not worry about those ions for a moment, but note how fuzzy the dictionary writer’s thinking was. To define a plasma like this is similar to calling a liquid ‘a very dense gas with fluid properties’. A plasma is certainly more like a gas than a liquid, just like a gas is more like a liquid than a solid, but it is still something else; a different state of matter.
I said that plasmas are more obvious than gases because they are usually highly visible. The Sun is a huge ball of plasma. Every flame contains some plasma, although the flames we usually encounter are fairly cool in plasma terms, so usually consist of a mix of plasma and gas. Just as a gas is what happens to a liquid if you continue to heat it past a certain point, so a plasma is what happens to a gas if you continue to heat it far enough.
As the gas gets hotter and hotter, the electrons around the atoms in the gas get more and more energy. Eventually some have enough energy to fly off and leave the atom behind. Most atoms have a natural tendency either to lose or gain electrons. Atoms that easily lose electrons do so, and end up as a positively charged ion. Atoms that easily gain electrons hoover up the spare ones from the positive ions and end up as negatively charged ions. Ions are just charged atoms with either electrons missing or electrons added. A substance that has been heated so far that its atoms become ions is a plasma.
Plasmas are very common once you consider the universe as a whole. After all, stars are pretty big objects. It has been suggested that up to 99 per cent of the universe’s detectable matter is plasma. In part this is because plasmas glow, so they are easier to spot. Although plasmas are gas-like, in not being hugely dense, they are very different from gases. For instance, gases are pretty good insulators, while plasmas are superb conductors.
Experiment – The state of custard
We usually think of materials changing state as a result of variations in temperature. Cool down water and it becomes ice. Heat up a piece of metal and it becomes molten (liquid) metal. But pressure can also have a dramatic effect on some materials. Thixotropic non-drip paints change between gel form (a gel is a malleable solid) and liquid when stirred. But the most dramatic and fun demonstration of the effect of pressure on the state of matter is provided by custard.
Mix custard powder with water so you get a thick yellow liquid. Pour some into a bowl. Now put your finger and thumb into the liquid a few centimetres apart and squeeze them together. The liquid becomes a dry powder under the pressure of your fingers. As long as you keep the pressure up, it will stay solid – you can easily lift it out of the bowl – but as soon as you relax the pressure it will return to liquid and drip from your fingers.
This quality makes it possible to walk across the surface of a pool of custard. To see this in action, visit www.universeinsideyou.com, select Experiments and click on Walking on Custard.
Enter the condensate
The fifth state of matter is not custard, but it is just as strange. On a good day, scientists can come up with impressively snappy terms. ‘Plasma’ is pretty good. So are ‘photon’ and ‘quark’. But all too often they come up with a name that no one in their right mind wants to say – try saying this one five times over very quickly. The fifth state of matter is a Bose–Einstein condensate.
This is a state down the other end of the temperature scale from a plasma. In fact, before we visit the condensate, it’s worth just briefly thinking about temperature. What is temperature? It’s how hot something is – fair enough. To heat things up we have to put energy into them. But what is happening as we do? The atoms or molecules in the material speed up. Even in a solid, atoms jiggle with energy. In a liquid they move about, while in a gas they positively rocket around the place.
When you use a thermometer to measure your body temperature (around 37°C), you are taking an average measure of the energy of movement in the particles that make you up. If you aren’t sure about there being a difference in energy just because something’s moving faster, imagine being hit by a tennis ball at 5 kilometres per hour, then at 500 kilometres per hour. The second one would hurt a lot more thanks to all that extra energy.
Unless you knew that temperature was about the movement of the atoms in a material, you might imagine that you could just cool things down indefinitely, getting colder and colder, as far as you liked, assuming your refrigeration mechanism was good enough. In practice, though, you can only slow down the atoms or molecules so much. Eventually they would stop. That temperature, unreachable in practice because quantum particles can never entirely stop, is absolute zero.
This ultimate low temperature is around –273.16°C. Scientists, however, often use a temperature scale that has the same size units as Celsius, but which starts sensibly with zero at absolute zero. This is the Kelvin scale, so 0°C is about 273 K on that scale. (For those who like pedantic detail, the units of the Kelvin scale are kelvins, with a small k, but the symbol is a capital K. Unlike Farenheit and Celsius there are no ‘degrees’ – so the freezing point of water is 273.16 K, not 273.16°K.)
When materials get close to absolute zero, they begin to behave very strangely. Some substances become condensates (technically there are two variants, Bose–Einstein and Fermionic, but let’s not worry about too much detail here). A condensate is a state of matter where the particles that make it up lose their individuality. This results in strange behaviours like superfluidity, where the substance has absolutely no resistance to movement. Superfluids climb out of containers of their own accord, because there is no resistance to the random movement of the molecules. If you start a superfluid rotating in a ring it will go on forever. Then there are superconductors, which have no electrical resistance.
The pièce de résistance of the condensate world is the way a Bose–Einstein condensate deals with light. Because the condensate is halfway between normal matter and light itself, it can interact with light in a strange way, slowing it to a crawl or even bringing it to an effective standstill. This weird mix of light and matter is called a ‘dark state’, a romantic name that well fits such an odd phenomenon.
Every kind of stuff
So that’s five states of matter. Up at the top, plasma, a collection of high-energy ions. Next a gas, then a liquid, then a solid. Finally, at the extreme limits of cold, the Bose–Einstein condensate. It’s easy to think of materials – stuff – as being rather ordinary and boring science. Yet there’s a remarkable amount going on in that individual hair.
Look close enough and you have molecules, made of atoms. As we have seen, each atom has its nucleus of protons and neutrons (apart from hydrogen, which is so small that its nucleus is just a single proton) and its surrounding cloud of electrons. And each of the particles in the nucleus is made up of a triplet of quarks. These simple building blocks are responsible not only for the relatively straightforward structure of your hair, but for all the complexity that goes into your body.
You are what you eat
But where did the components of your body come from? Where were those atoms before they were incorporated into you? In previous centuries they were drifting around the planet, getting involved in all manner of reactions. There’s an awful lot of carbon in your body, for instance. Where did it come from? Plants and animals, which in turn got theirs from other plants and animals. And if you go along the chain far enough you’ll hit a vegetarian. So ultimately all that carbon came from plants. But where did they get it?
The air.
Plants have the wonderful ability to build themselves largely from air. We’re used to carbon dioxide being treated as a bad guy because of its role as a greenhouse gas, but bear in mind that most of the carbon that gets incorporated into plants comes from the carbon dioxide they take out of the atmosphere. That’s just as well, as they then pump out the waste oxygen, and that’s the only reason we can breathe.
So prior to being in other animals and plants, some of your atoms were in the air. Some came from the ground and from water. Go back far enough, and many of them will have spent time in other people in history. There are so many atoms in a person (7 × 1027) that after a while, many of them will be recycled in other human beings. Your body contains atoms from kings and queens, noble warriors and court jesters.
This is subtly different from the suggestion that every breath you take contains an atom or two that was breathed by Marilyn Monroe. The atmosphere moves around with sufficient vigour to mix those breaths into the whole and get the odd atom into your next intake of fresh air. But the atoms that made up Marilyn haven’t had time to spread around the world and get into everyone’s body. Some people will have them, but not everyone. Go a few hundred years forward in time, though, and it will be pretty certain that molecules of Marilyn will be in every person’s body.
Components that pre-date the Earth
The atoms inside you have been circulating around on Earth since life began, well over three billion years ago. Fossils can be used to trace life back in rocks that were formed around 3.2 billion years ago, while the date can be pushed back a few hundred million years more on the basis of chemicals that suggest the existence of life. But before then, the atoms were still there. They didn’t appear out of nowhere. The atoms that make you up were present when the Earth was formed 4.5 billion years ago (apart from a few that arrived since on meteors from outer space).
Before that they floated for aeons through space. Some have been around since the beginning of the universe. According to the Big Bang theory, our best idea of how the universe began, all of the hydrogen in the universe and some of the helium and lithium was created when the remnants of the Big Bang that formed the universe cooled down enough to stop being pure energy and formed matter. So the hydrogen in the water and organic molecules in your body date back to the very beginning of the universe.
After a while, some of this hydrogen clumped together, pulled by gravity, and formed stars, which burn in their youth by converting hydrogen, the lightest element, into the next element, helium. When most of the hydrogen is used up, helium too can be consumed, working up the elements all the way to iron. And this is where elements like the carbon and oxygen that are so important for life were forged.
Later still, some of those stars would become unstable and detonate in catastrophic explosions called supernovas. Ordinary stars don’t have enough energy to make the elements that are heavier than iron, but supernovas have so much oomph that they can create elements all the way up to uranium, the heaviest of the naturally occurring elements.
This means that, quite literally, you are stardust. The atoms within the hair you hold, and within every part of your body, either came from the Big Bang – so are 13.7 billion years old – or from a star, which would make them between seven and twelve billion years old. The components of your hair – and every other part of you – are truly ancient. We tend to think of the universe explored by astronomers as very distant and not really connected with life on Earth. Yet every atom inside you was once out there, once part of the wider cosmos.
A sprinkling of stardust
This makes you rather special. Atoms are a rarity in the universe. There really aren’t many of them out there. This might seem unlikely, considering all the stuff we see around us, let alone all the stars and galaxies in the universe, but it’s a big place. It has been estimated that there are around 1080 atoms in the observable universe, that is, all of the universe it’s possible to see. Distances in space tend to be measured in light years, the distance light covers in a year. As it travels around 300,000 kilometres per second, that makes a light year around 9.5 trillion kilometres. And the visible universe is about 90 billion light years across.
We say ‘the visible universe’ because no one is sure how big the universe is. However, there’s quite a lot of evidence that suggests the universe came into existence about 13.7 billion years ago. So we can only see light that has been travelling for 13.7 billion years (slightly less, actually, but let’s not worry about that). If everything stayed the same, that would make the visible universe about 27 billion light years across – but the universe has been expanding since it began. So the point the light set off from 13.7 billion years ago is now around 45 billion light years distant.
The universe is so big that if you distributed all the atoms in it evenly throughout space, there would only be one oxygen atom in about every 6,250 cubic metres. Just think of that in terms of your body. By far the biggest component of your body by mass is water. And most of water’s mass is oxygen – so the biggest atomic component of your body is oxygen, which accounts for about 65 per cent of your mass. So, if all the matter in the universe was nice and evenly spread out, to provide the oxygen in you would require the contents of over 9 × 1030 cubic metres of space. A cube twenty million kilometres on each side; that’s more than 50 times the distance to the Moon.
Think about that hair from your head once more. People take great pride in working out their genealogy over a few generations. If a country house has been owned by the same family for 400 years they consider themselves something special. But that hair you are holding has contents harvested from across space, with some of its atoms going all the way back to the Big Bang, and all of them well over five billion years old. That’s what I call having ancestry.
Your hair, as you discovered earlier, is dead. But now it’s time to move over to signs of life. And what’s more suggestive of life than blood?