

 Healthy plants result from a healthy soil food web.
Healthy plants result from a healthy soil food web.
 Unlike chemical fertilizers, natural fertilizers foster the health of the soil food web, which builds all-important soil structure.
Unlike chemical fertilizers, natural fertilizers foster the health of the soil food web, which builds all-important soil structure.
 Good natural sources of nitrogen include bat guano, blood meal, corn gluten meal, and even human hair and urine.
Good natural sources of nitrogen include bat guano, blood meal, corn gluten meal, and even human hair and urine.
 Biofertilizers are living organisms that are added to the soil to promote plant health. These include nitrogen-fixing bacteria, phosphate-solubilizing bacteria and fungi, and mycorrhizal fungi.
Biofertilizers are living organisms that are added to the soil to promote plant health. These include nitrogen-fixing bacteria, phosphate-solubilizing bacteria and fungi, and mycorrhizal fungi.
 I provide some organic fertilizer recipes designed for annuals, vegetables, lawns, perennials, trees, and shrubs and describe the best ways to apply these fertilizers.
I provide some organic fertilizer recipes designed for annuals, vegetables, lawns, perennials, trees, and shrubs and describe the best ways to apply these fertilizers.
NOW THAT YOU know how plants obtain the essential nutrients that are in fertilizers, it’s time to discuss which are the best to use and when. No two gardens are alike, so the choice of fertilizers to use has to be an individual one based first on sound soil testing. The rest is up to the garden and the gardener. It is no longer acceptable to simply buy a package of fertilizer based on a picture of the plants you want to feed.
For starters, use natural fertilizers, not synthetic ones. Some would argue that it can’t possibly make a difference to a plant if its nitrogen and other essential elements come from the remains of another plant or an animal or if the fertilizer is synthesized in a factory out of chemicals. These nutrients have to be in inorganic form for uptake. Synthetic fertilizers do work in the same way as natural ones once inside the plant. And, for the most part, the organic matter in natural fertilizers has to be reduced to inorganic compounds before nutrients can enter a plant. The big difference and the reason organics and natural fertilizers are better, however, is because they feed the soil food web, which makes soil structure. Inorganic fertilizers generally do not build or help sustain soils.
Chemically, the implication of using synthetic fertilizers is clear. They can tie up essential nutrients, quickly change the pH, and adversely affect osmosis because of their high concentrations. The ultimate repercussions are symptoms of poorly nourished plants. Synthetic fertilizers also have a negative impact on the soil biota. Once the soil food web organisms are affected, either directly or indirectly, the soils lose the structure these organisms create. Bacterial slime and fungal hyphae initially stick and weave soil particles together, creating pore spaces, reservoirs for air as well as water and places for the smaller organisms to hide from predators. Tunnels and burrows further increase the air- and water-holding capabilities of the soil. Dead organisms contribute to the carbon supply, which supports living organisms. Because synthetic fertilizers either kill or repel bacteria and fungi or don’t contain the organic bulk that natural fertilizers do, plants get fed, but soil structure doesn’t get built.
A reduction in mycorrhizal fungi means the soils don’t get the carbon these fungi deposit via their coating, glomalin. In addition to providing nutrients, mycorrhizal fungi help protect plants. There is chitin, a nitrogen polymer, in the sheaths around roots that is not only a source of nitrogen but keeps parasitic nematodes in check, and some produce antifungal chemicals to ward off competition. By the same token, the antibiotics some microbes produce are missing once these organisms are killed by the use of synthetic fertilizers.
These symbiotic relationships between plants and soil microbes have been maintained for some 420 million years. About 60 percent of the carbon produced by plants during photosynthesis is used to synthesize many different exudates that influence pH, the type and diversity of the soil organisms, plant nutrient uptake, plant defense, and soil structure. These carbon-rich root exudates support Rhizobia and Frankia that fix nitrogen.
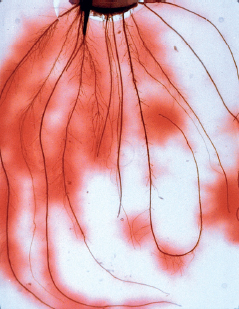
Plant exudates can adjust pH and nutrient supply, and even affect soil quality, by supporting the organisms of the soil food web.
It’s not just the animals in the soil with which plants have developed relationships. There is one with the soil itself. Plants exchange hydrogen and hydroxyl ions to change the pH so they can get the nutrients they need. In short, plants are capable of modifying the soil chemistry. Some plants, such as grasses, can produce siderophores that help to unlock iron from the soil.
Organic fertilizers are, by definition, full of organic matter, which supports the creation and maintenance of good soils and healthy, diverse soil food webs. These fertilizers contain their nutrients in bulk. Thus, their use also increases the cation exchange capacity (CEC) of soil because it is adding organic matter that holds a charge. Applying organic fertilizer is like constructing a condominium for the organisms that fix nitrogen and mineralize and cycle plant nutrients, ultimately making them available in the very same ionic form as do chemical fertilizers.
Soil structure is important to good gardening. Lose it and conditions become increasingly anaerobic. Plants have a hard time taking up nutrients in such conditions, as we learned in the last chapter. The populations of animals in the soil expel carbon dioxide. Plant roots and beneficial organisms in the rhizosphere around them need oxygen in order to survive. Unless this carbon dioxide is removed, it will react with water and minerals in the soil and create acidic conditions. Some of the carbon dioxide dissolves in water and becomes carbonic acid, which, though a weak acid, still produces hydrogen ions, thus lowering the pH. This also can lead to anaerobic conditions. When this happens, nitrogen fixation diminishes and there is a whole cascade of effects that have negative impacts on plant nutrition.
The addition of organic matter in natural fertilizers helps create a structure in which the carbon dioxide from the microbial activity in the soil is not trapped. This structure also ensures that the proper moisture content, which is critical for the uptake of nutrients, can be much more easily maintained. In fact, if you have sandy soils, using natural fertilizers is the only way to hold in water and may be the only way to really provide adequate nutrients to plants.
Synthetic fertilizers usually contain much higher percentages of nitrogen, phosphorus, and potassium than natural fertilizers, which are derived from plant and animal remains and rock dusts. Thus, the nitrogen content may be as high as 60 percent in some chemical fertilizers, whereas almost all organic fertilizers have about 12 percent nitrogen. However, macro- and micro-arthropods (such as springtails and fungus-feeding mites) and worms, in particular, shun areas where there are synthetic fertilizers. They either don’t do well with these high concentrations or their food sources disappear. This further degrades the soil structure and the production of humus. In addition, mycorrhizal fungi and nitrogen-fixing bacteria do not form symbiotic relationships when there are high levels of nitrogen and phosphorus. Mycorrhizal fungi are the largest single source of carbon in soils, and nitrogen-fixing bacteria are a free source of nitrogen, a macronutrient most often in short supply. So, by maintaining a healthy soil food web, gardeners can get carbon, nitrogen, and other nutrients for their plants for free.
When organic fertilizers are applied, the natural substances are broken down into organic compounds by microbes in the soil. Eventually, humus, the end product of composting, is created. This is what good soil is all about, and you can’t develop humus without organic matter and microbes. Natural fertilizers are more complete fertilizers. Not only do they provide bulk, which can be used by the soil food web, they also supply many more nutrients at one time, not just nitrogen, phosphorus, or potassium.
Other arguments can be made for the use of natural fertilizers. For one thing, making chemical fertilizers is an extremely energy-intensive activity. These products also have to travel great distances to get to market, which adds to their environmental footprint. More important, many synthetics are anions because farmers need to get their plants fed quickly and need fertilizers that are instantly soluble in water. These anions are easily leached out of the soil and cause serious pollution as they run off into water bodies. Finally, it is often impossible to determine exactly what is in commercial synthetic fertilizers. In the United States, each state has its own labeling laws, and many allow the use of fillers without having to identify their source.
The runoff from thirty-one states and two Canadian provinces enters the Mississippi River and flows to the Gulf of Mexico. When farmers and gardeners in these regions apply excessive amounts of nitrogen and phosphorus fertilizers in soluble form, these nutrients leach into the river. As a result of this pollution, a huge dead zone forms each summer along the coasts of Mississippi, Louisiana, and eastern Texas.
In the spring, warm freshwater runoff from the Mississippi creates a barrier layer atop the Gulf of Mexico. This barrier cuts off the saltier water below from oxygen in the atmosphere. Nitrogen and phosphorus pollution in this freshwater layer cause huge algal blooms to form in the Gulf, shown in red and yellow in the photo. When the algae die, they sink into the saltier water below and are decomposed, which uses up the limited oxygen in that lower layer. This lack of oxygen, a condition known as hypoxia, causes fish and other organisms to avoid the area or to die in massive numbers. The cooler temperatures of winter disrupt the barrier layer, but the cycle starts again each spring.
Although farmers usually get all the blame for the excessive use of synthetic fertilizers, gardeners also play a role. In fact, studies have shown that gardeners use three times more synthetic nitrogen per acre than do farmers.
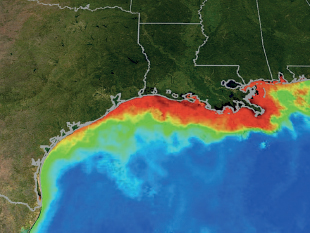
The red and yellow regions are dead zones in the Gulf of Mexico caused by pollution from runoff.
There are arguments against the use of natural fertilizers, too. The first is that you can’t feed the world using just organics. For our purposes, however, the argument doesn’t really apply. Gardening is a hobby, and it simply does not make sense to use chemicals that degrade the soil and greater natural environment. If you don’t consider your garden a hobby, because the food from it is a necessity, then all the more reason to ensure you are not poisoning your family or reducing the ability of your soil to produce.
The strongest argument against the use of natural fertilizers over synthetic ones is that organic fertilizers are not immediately available to plants when they are applied, as are synthetics. This is true. (Although the reverse is also true: synthetics are available too quickly and not for long periods.) Most natural fertilizers are slow-release fertilizers. However, this situation can be overcome in most cases by some careful planning and preseason application of natural fertilizers or by the use of natural fertilizers that are actually readily available after application. This argument only applies at the start of a natural system. Once slow-release natural fertilizers are in the soils for a while, the soil food web releases the nutrients.
A fertilizer, by definition, is any soil amendment that can guarantee a certain percentage of nitrogen, phosphorus, and potassium and sometimes other essential nutrients. (In Australia, for example, it is nitrogen, phosphorus, potassium, and sulfur). Laws require that the percentages of these elements be listed on fertilizer packages. This is why every fertilizer package carries the nitrogen–phosphorus–potassium (N–P–K) trilogy on its label. It is also why many people don’t consider compost or manures to be fertilizers. These are instead classified as soil amendments because in most cases the amounts of nitrogen, phosphorus, and potassium vary from batch to batch and are usually not measured for labeling purposes.
Most gardeners think the N–P–K trilogy represents the percentage by weight of nitrogen, phosphorus, and potassium in the container. That’s close, however, the phosphorus on the label is really the percentage of phosphorus pentoxide (P2O5) and the potassium the percentage of potassium oxide (K2O). This has to do with the way chemists used to measure the phosphorus and potassium. To make the conversions for a pure N–P–K trilogy, you need to multiply the number for phosphorus by 0.44 and that for potassium by 0.83 to determine the actual weights of phosphorus and potassium.
Natural fertilizers are derived from plant and animal by-products as well as rock powders. Although there are as many commercial brands available as there are hosta varieties, they can be grouped into dry fertilizers and liquids. Examples of dry fertilizers are blood, soybean, fish, cottonseed, and alfalfa meals, as well as bat and bird guano and rock phosphate. These are applied either on the soil surface or in the root zone when planting. They can also be mixed into soil when starting a garden. Most are decayed slowly by the soil food web. Liquid fertilizers include things like fish emulsions and kelp extracts. These work faster than most dry fertilizers (bat guano and fish meals are, perhaps, exceptions), provided there is a healthy soil food web to cycle them. Liquids are normally applied to roots and, in some circumstances, sprayed on leaves as a foliar feed. Plants, however, require far more macronutrients than they could ever absorb via leaves, and many nutrients are not mobile once inside plants, so foliar feeding is of limited value.
This dry versus liquid division is good for deciding on an application method. However, now that we know something about the fourteen essential mineral elements, it makes sense to group natural fertilizers by the major nutrient they provide. Some natural fertilizers list more than N–P–K numbers. Those that are used for the other essential nutrients will usually list these and their percentages on the label. You now know what to look for.
It is important to remember that the microbes in the soil decay most natural fertilizers, although some become available due to weathering. The right environment increases the cycling of nutrients by microbes. Warmer temperatures (to a point) will speed up the microbial process if there is adequate moisture. Therefore, the length of time natural nutrients will be available after application varies.
Alfalfa meal (N–P–K 2–1–3). This is the very same stuff that sustains pet rabbits and horses. Alfalfa meal is a good all-purpose source of nitrogen and contains trace elements as well. Alfalfa meal feeds bacteria and fungi and is usually covered with protozoa, each of which can cycle 10,000 bacteria a day into plant-usable ammonium. It is available for cycling into nutrients by microbes for about 1 to 4 months.
A few caveats are in order. First, because alfalfa is a plant, the meal does contain natural growth hormones, so it can be overused. Use it in the early life of a plant to stimulate growth. The other warning is that alfalfa meal can sometimes contain seeds, so examine it before buying or using it to prevent unwanted weeds. Of course, this can also be seen as an upside: the seed will grow and attract endomycorrhizal fungi, which help to add carbon to your soils.
Bat guano (N–P–K 10–3–1). Bat guano (feces and urine) is what many of the world’s farmers used before synthetic sources of nitrogen were available. It is not only rich in nitrogen, but the nitrogen is in a soluble and readily available form. It can be used at the start of the season or when a quick pick-me-up is needed. It is great for the first-time natural garden when there hasn’t been enough time for fertilizers, mulches, and composts to decay and provide sufficient plant nutrients to garden plants. Bat guano is applied in powder form or mixed with water and then applied. It will last from 4 to 6 months.
There are three warnings to consider before using bat guano. If too much is used, it can burn plants, meaning that its presence can pull water out of the cells by osmosis. Next, there are two kinds of bat guano. One is full of nitrogen, and the other is full of phosphorus with much less nitrogen. Read the labels. Finally, bat guano can be expensive, but it lasts for 4 to 6 months. Make sure any guano you buy has been harvested in a sustainable way.
Blood meal (N–P–K 12–0–0). This meal is made from dried blood collected during the processing of livestock, and it is very high in nitrogen. It doesn’t get better, so to speak, because not only is blood meal high in nitrogen, but this nutrient is readily available. Thus, as a fast-acting, natural fertilizer, blood meal that can be used to counter the argument that natural fertilizers are released too slowly. The nutrients in blood meal are released for about 1 to 4 months.
Here are the warnings. With such a high nitrogen content, blood meals can burn plants. It also has an unpleasant odor. Finally, blood meal will attract animals, such as dogs and cats. On the plus side, deer (and moose for those who have them) hate its smell and normally avoid it like the plague. They smell the blood and think a predator has made a kill.
Cottonseed meal (N–P–K 6–0.4–1.5). This is a slow-release, high-nitrogen fertilizer that lasts around 4 months. Cottonseed meal also contains trace elements, such as zinc, copper, manganese, and molybdenum. It is slightly acidic and good for use with acid-soil loving plants, like rhododendrons, azaleas, camellias, and blueberries.
The downside to cottonseed meal is that cotton growers usually use tremendous amounts of pesticides, and these residues can be found in this fertilizer product. Therefore, it is best not to use cottonseed meal on vegetables and fruit crops. There is also the issue of cotton being a genetically modified organism (GMO). This is one crop that may be glyphosate-ready and would not be considered organic.
Corn gluten meal (N–P–K 9–0–0). This is another high-nitrogen natural fertilizer. Corn (maize) gluten is the by-product of the manufacturing of corn syrup. It is effective over a period of 1 to 4 months, depending on rainfall.
The downside, besides expense, is that corn gluten prevents root hairs from developing on germinating seedlings. As you now know, no root hairs, no plants, so you can’t use it when you are trying to germinate seeds. Corn gluten meal is much better for use in established lawns and perennial gardens where seeds are not expected to germinate. Corn gluten may come from GMO plants, for those who are concerned about the possibility of ingesting Bacillus thuringiensis or who maintain a totally organic garden.
Feather meal (N–P–K 7 to 12–0–0). A by-product of the poultry industry, feather meal is a high-nitrogen plant food. However, it releases the nitrogen much more slowly than bat guano or blood meal. In fact, this is a really slow release natural fertilizer, because feather meal is full of a protein called keratin, which is complex and requires a bit more microbial digestion to fully decay it. Feather meal can continue to be a source of nitrogen for 6+ months.
The use of feather meal may attract dogs, raccoons, and bears.
Fish emulsion (N–P–K 5–2–2). This is heat- and acid-processed fish parts that results in a soluble and highly concentrated nitrogen fertilizer that has a bit more balance of the essential nutrients that the others. Fish emulsion is full of micronutrients, too, as fish eat lots of things that contain micronutrients. Fish emulsion is soluble and thus quick acting. It needs to be diluted because of its concentrated nutrients. This fertilizer lasts from 1 to 4 months.
These liquids usually smell like rotting fish. Do not (as I once did) apply it to the lawn the same day you want to use it for entertaining. Fish products attract flies, too, which help in the decay process. They can attract bears (at least here in Alaska), and some dogs love to roll in grass after it has been applied. Things usually return to normal within a few days.
Fish meal (N–P–K 10–6–2). This meal is ground fish parts that are heated and dried. In addition to nitrogen, fish meals are a good source of phosphorus. Like the other fish-based fertilizers, fish meal lasts from 1 to 4 months. Fish meal is not readily soluble and not as quick starting as other fish fertilizers, but it is a decent phosphorus source, which the others are not.
Fish meals smell for a few days, although they do not smell as strongly as fish emulsions. They also can attract flies, bears, and dogs. Fish meals are heat processed, which results in a loss of some of the enzymes and other proteins, vitamins, and even micronutrients that remain available in the other fish products.
Fish powder (N–P–K 12–0.25–1). Fish powder is another heat-processed fish material, only this has a highly soluble form of nitrogen. Because fish powder is water-soluble, it can leach out of soils easily. It is fast acting and used up within 1 month under normal conditions. Like blood meal, fish powder is another fertilizer that acts almost as quickly as synthetic fertilizers.
Hydrolyzed fish (N–P–K 4–2–2). This product is fish that have been enzymatically digested in large tanks instead of being heat processed. The remaining liquid retains many more proteins and other compounds than if heated. This medium-acting natural fertilizer lasts up to 5 months.
The downside is that hydrolyzed fish can be relatively expensive.
Human hair (N–P–K 18–0–0). Wow, talk about a lot of nitrogen! For every 7 pounds of hair, there is around 1 pound of nitrogen. Here is a natural fertilizer that exceeds the normal percentage for organic fertilizers, with a nitrogen content closer to a chemical lawn food. You won’t find this bagged and on the market, but you can get it for free. Hair, fortunately for those of you who still have yours, is not readily soluble and does not readily decay, so the high nitrogen content doesn’t harm the soil food web. In fact hair is a very slow-release source of nitrogen, lasting from 1 to 2 years.
Human urine (N–P–K 15–2–2). Urine is another fertilizer that has a relatively high nitrogen content for natural fertilizers. It gets my vote for the first fertilizer ever used. It is sterile when first produced and contains urea, which when pure has an N–P–K value of 46–0–0. Soil bacteria quickly convert urea into ammonium. If the soil pH is acidic, this is the form mostly taken up by plants. If the pH is basic, which encourages nitrifying bacteria populations, the ammonium is converted into nitrates for uptake. Either way, urine can burn tender plants (and microbes) if not diluted with at least 8 parts water to 1 part urine. (However, from 60+ years of personal experience, I can tell you there is no problem with occasional application to trees and shrubs, especially when no one is looking.) Used undiluted, it is great for application to compost piles. One application will last for 1 or 2 weeks.
The downside of human urine is concerns about the possible presence of heavy metals, such as mercury, as well as antibiotics. Do not use urine on food crops.
Soybean meal (N–P–K 7–2–1). Soybean meal is one of my favorite all-purpose fertilizers because it supplies lots of carbon as well as nitrogen, doesn’t smell, stores well, and works in a spreader or when broadcast by hand. It quickly attracts both fungi and bacteria, which slowly release its nutrients for 3 or 4 months.
On the downside, soybean is one of the biggest GMO crops. The insertion of bacterial genes into a plant genome results in a protein molecule being produced that has never been in that plant or in food derived from it. These may require special microbes to break down, both in the soil and in animals. Some argue that this is the cause of an increase in soy allergies and autoimmune and digestive problems. Studies on cattle, poultry, and swine indicate that greater caution should be used with GMO crops. Now that you know how plants eat, you have the tools to follow the debate on this issue, which includes deciding whether to use GMO-containing fertilizers.
Chilean nitrate (N–P–K 16–0–0). This is a quick-acting, fully soluble mineral fertilizer that has percentages of nitrogen over the norm for natural fertilizers. It can be used to boost nitrogen without increasing anything else. While not usually allowed under certified organic systems because it is not technically organic, Chilean nitrate is a natural nitrate mined in the desert of northern Chile. Some organic certifying agencies allow its use in limited quantities, particularly where soils remain cool so microbial mineralization of nitrogen is low and slow and a quick release is needed. Natural nitrate soda is fast acting because it is soluble immediately and does not require microbial activity to convert it into ions. It is mobile in the soil and will only be readily available for 1 or 2 weeks or until the next good rain.
Natural nitrate soda does contain 26 percent sodium, which is fine in small quantities. This fertilizer should not be used where there is already high sodium content in soils, such as in deserts and dry regions. Sodium can also react with clay, which makes it difficult to build soil structure and aeration. This is one natural fertilizer whose use does not improve soil tilth (structure), so Chilean nitrate should not be the sole source of a garden’s nitrogen. It is best to limit its use to start a new garden and at the beginning of the season, especially in gardens where it takes a long time to warm up.
Animal bone meal (N–P–K 3–15–0). This fertilizer is made by steam processing and grinding bones. The phosphorus in bone meal is very readily available and lasts 1 to 4 months.
Unfortunately, bone meal does not work well as a source of phosphorus unless the pH is below 7. You have to read labels. Some brands list the percentage of phosphorus and others phosphate, which is the norm. It also may attract animals until the attractant odor disappears 1 to 2 weeks after application.
Bat guano (N–P–K 3–10–1). Guano (the feces and urine of cave-dwelling bats) comes in a high-nitrogen form and one with lots of phosphorus. Because phosphorus is quickly tied up in soil, high-phosphorus bat guano will last 1 to 4 months. The best releasers of this nutrient are mycorrhizal fungi. Too much phosphorus, however, limits their presence, so go easy and make sure your laboratory knows you rely on mycorrhizal fungi when they develop recommendations based on your soil tests.
Unless you are starting a garden and have not had time to prepare it a few months ahead of planting, use this fertilizer carefully and only when a testing laboratory suggests guano as a source of phosphorus.
Colloidal rock phosphate (N–P–K 0–2.5–0). Sometimes called soft rock phosphate, this material consists of clay particles surrounded by phosphate. The clay helps to improve the soil’s cation and anion exchange capacities. This stuff lasts years, slowly releasing phosphorus that makes its way to the roots via mycorrhizal fungi or by diffusion. Some gardeners claim that because of the way phosphorus is taken up into plants, colloidal rock phosphate actually works better the second and third years. It remains available for 3 to 5 years, depending on rainfall and watering.
Placement is key with rock phosphates. They work best when placed where roots will intercept the particles.
Crab shell meal (N–P–K 2–3–0). This is a good source of phosphorus, if you can find it. In addition to the nitrogen and phosphorus content, crab shell meal also contains lots of calcium and trace elements. It also contains chitin, making it a great fungal food. Chitin can also control high populations of nematodes.

Nests of the Peruvian booby are made of guano.
Greensand (N–P–K 0–0–7). Often called New Jersey Greensand or glauconite, this mineral was formed on ancient sea beds and contains more than thirty elements, including calcium, magnesium, and iron and other micronutrients, as well as potassium. It has to be weathered and is slow to release nutrients, so it lasts 2 or 3 years.
Wood ashes (N–P–K 0–1–3). Wood ashes or pot ash, as they were referred to in the old days, have been in use for centuries as a source of potassium. They are free for those with a fireplace, but care must be taken not to use ash from treated charcoals or woods, as harmful chemical residues may remain. Ashes are alkaline and will increase pH, so they should not be used in soils that are already alkaline. They last 1 to 2 months.
Sulfate of potash (N–P–K 0–0–2). This mineral salt is often labeled Sul-Po-Mag. It is very soluble in water and contains 23 percent sulfur, 22 percent potash, and 11 percent magnesium. Some gardeners do not consider it to be organic, because it does not add bulk to the soils. The solubility of sulfate of potash makes it very quick acting, and it lasts only about 2 months.
Calcitic limestone (N–P–K 0–0–0). This material is from sedimentary rock that contains calcium carbonate (CaCO3). A slab of limestone will last thousands of years, but when powdered and exposed to water and carbon dioxide, which form an acid, it breaks down. How long the calcium ions remain in the soil has much to do with its pH and organic matter and clay contents. It is more important to know that you can only raise pH a point or so per growing season. The best time to apply is in the autumn for the following growing season.
Dolomitic limestone (N–P–K 0–0–0). In addition to providing calcium, dolomite or dolomitic limestone (CaMg[CO3]2) is added to soils as a magnesium source because it contains about 10 percent of this element. It tends to bind soils and too much magnesium impacts the uptake of other nutrients, so make sure your soils test shows you actually need more magnesium. Again, the efficacy and duration of limestone in the soil is related to the existing pH, water, and carbon dioxide, another great example of why testing is so important.
Shrimp shell meal (N–P–K 5–8–15). This meal is made by grinding up the heads and shells of shrimp. Shrimp shell meal also contains lots of trace elements, as you would expect from a creature that lives in the ocean. In addition to phosphorus and potassium, it also contains about 15 percent calcium and about 20 percent chitin. Shrimp shell meal is a slow-release fertilizer that lasts 3 to 6 months.
Kelp meal (N–P–K 0–0–0 to 1–0–4). Kelp meal is made from kelp, a type of seaweed. Seaweeds contain up to sixty elements, including all the trace minerals that plants need. Kelp meal also contains natural plant growth hormones, too. The nutrients are generally available about 1 or 2 months after application, and they are then slowly released during the 4 or 5 months that it takes the meal to decay.
The negative is that, while often very effective for hydroponics, many of these nutrients are already in the soil. Although trace minerals are essential, they are needed in very minute quantities. In addition, it is important to make sure the kelp is harvested sustainably.
Kelp powder (N–P–K 0–0–0 to 1–0–4). Kelp meal is further ground to make kelp powder. This makes it more soluble and thus more readily available to microbes, which quickly make the nutrients available to plants. It lasts for up to 1 month.

Kelp on the beach of Chowiet Island, Alaska. Kelp is full of micronutrients.
Liquid kelp (N–P–K 0–0–0 to 1–0–4). This is another good source of micronutrients that are instantly available to plants when applied. Most brands of liquid kelp are made by enzymatically digesting kelp, which preserves more of the plant’s growth hormones than the other processes used to convert kelp. It lasts 1 to 3 weeks in the soil.
More and more gardeners are using live agents to help produce plant foods. Again, while not fitting the current legal definitions of fertilizers because it is difficult or impossible to quantify their N–P–K contents, there are plenty of microorganisms that produce or are intimately involved in the production of plant nutrients. These go beyond the decaying and cycling microbes. Microbes described as biofertilizers produce nutrients.
Rhizobia and Frankia (for nitrogen). Both of these bacteria are symbiotic nitrogen fixers. They can produce a lot of useable nitrogen. The use of Rhizobia to grow legumes (such as soybeans, locust trees, and wisteria) and to produce nitrogen for soils is a well-established practice. Frankia were discovered much later and their use is still developing. Remember, there is incredible specificity between plant and microbe when it comes to nitrogen-fixing diazotrophs. This has to be taken into account when trying to employ them in soil.
Azotobacter and Azospirillum (for nitrogen). These two bacteria genera are free-living and produce nitrogen without entering into a symbiotic relationship with plants. Azotobacter and Azospirillum are frequently used when growing cereal crops. Researchers are working to develop mixtures for gardening use.

These tiny round root nodules are filled with specialized bacteria that capture nitrogen from the air and trade it with the legume for sugars the plant produces. The pinkish color comes from a compound similar to the hemoglobin in red blood cells.
Phosphate-solubilizing bacteria and fungi (for phosphorus). Certain types of nonsymbiotic bacteria (Bacillus megaterium var. phosphaticum, Bacillus subtilis, Bacillus circulans, Pseudomonas striata) can free up insoluble phosphorus and either make it directly available to plants or put it into the diffusion stream that brings phosphorus to plants. They are called phosphobacterins.
Similarly, certain fungi (Penicillium species, Aspergillus awamori) also free up phosphorus. They act indirectly on the insoluble phosphorus by producing organic acids as they go about their business. These acids break the bonds that tie up phosphorus in the soil.
Mycorrhizal fungi (for phosphorus, copper, zinc, molybdenum, and nitrogen). These fungi form mycorrhizae with plant roots. In return for the plant exudates that supply carbon (which fungi cannot make), mycorrhizal fungi obtain phosphorus from the soil and make it available to plants. Their long hyphae extend root surfaces up to hundreds of times, so interception for this and other nutrients is high. Mycorrhizae are the norm in the natural world, with over 90 percent of plants entering into such a relationship.
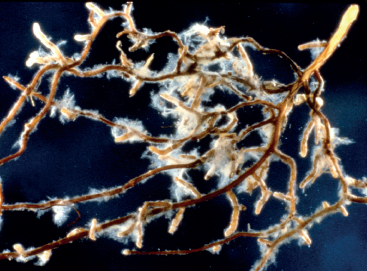
Mycorrhizal fungi may provide as much as 80 percent of a plant’s phosphorus and 60 percent of its copper along with other essential nutrients. This is Suillus infecting a pine root.
Some experiments have suggested that these fungi can deliver 80 percent of a plant’s phosphorus, 60 percent of its copper, 25 percent of its nitrogen, 25 percent of its zinc, and 10 percent of its potassium. This is free—not only economically, but work-free. Mycorrhizal fungi are the reason trees do so well with so little care.
Plant growth promoting rhizobacteria (PGPR). Rhizobacteria are root-colonizing bacteria that form symbiotic relationships with plants. The most well-known species is Pseudomonas fluorescens, but PGPR are actually a whole host of bacteria that aid in the synthesis of nutrients, positively influencing root growth and thus plant nutrition. The major function studied so far is the way these bacteria help mycorrhizal fungi with obtaining phosphorus, perhaps helping the fungi break bonds. Some PGPR produce alkaline phosphatase, an enzyme that breaks down phosphate bonds.
Compost (N–P–K varies, but low). Compost is technically not a fertilizer, but rather a soil amendment. However, compost is full of microbes and humus, and it provides a great environment for the microbial activity needed to cycle or decay natural materials into nutrients. Compost does contain nutrients because organic matter and any clay in the compost will have an impact on its CEC. Many would argue it should be tested for nutrients along with microbial content so that the gardener can have an understanding of what is being applied and how its application might impact soil laboratory recommendations.

Earthworm castings concentrate nutrients, which is why they are so popular with organic gardeners. They contain 10 times more potassium, 5 times more nitrogen, 7 times more phosphorus, 3 times more magnesium, 1.5 times more calcium, and 1.4 times more humus than the soil that went into the worm.
A big caveat is that compost must actually be composted. Anything that has not gone through the complete process, and many home compost piles never do, is not compost. Partially composted material can create problems, including tying up nitrogen, as the composting process is completed next to the plant instead of in a pile.
Earthworm castings (N–P–K varies, but high). Worm castings, another “almost fertilizer,” but this time because it’s next to impossible to supply a guaranteed N–P–K analysis, also contain a lot of microbes. The worms ingest organic matter, but what they are really after are bacteria, protozoa, and fungi, some of which they digest. They process the rest of the material into castings with a higher concentration of organic matter and great N–P–K, as well as calcium, copper, zinc, and other minerals, than in the source material.
Manures (N–P–K varies). Technically not fertilizers, livestock manures in the modern age have to be carefully assessed to determine what they contain in the way of antibiotics, hormones, and medicines. These often do not break down during the composting process. The use of manures can lead to the buildup of salts and heavy metals. In addition, if they are not composted properly, manures can be the source of E. coli. In the home garden, manures must be completely composted for at least 72 hours at a minimum of 131°F (55°C) before use.
Lots of things affect the availability of nutrients from organic fertilizers, and all of them relate to the ability of the minerals to be decomposed to their ionic forms. This decomposition is usually a direct result of the activity of organisms at the base of the soil food web: bacteria, Archaea, and fungi.
The temperature of the soil can make a difference in availability because the microbes that release nutrients are generally more active at warmer temperatures. In addition, you should ensure that your soil’s pH is in the right zone to maximize the numbers of unencumbered ions so that as few as possible are rendered unavailable to plants. However, the number one rule for maximizing the availability of fertilizers is to know what nutrients are already in your soil and as much as possible about its characteristics. Have a soil test done before you set up a feeding program. Retest the soil at the end of the season or the start of the next (or as otherwise advised by your laboratory) to see how things are trending. Thereafter, test garden soils every 2 or 3 years and adjust your program accordingly.
Now that you know something about how a plant eats, the next step might be to make your own natural fertilizers. Sure, you can buy all you need in commercial mixes. But how difficult can it be with only fourteen mineral elements needed? Besides, if a plant can take these and make millions of different kinds of molecular compounds, we should be able to make a few simple recipes ourselves.
There are several ways to make your own plant foods. Each is dependent on what kind of gardens you have and what kind of gardener you are, as well as the sources of supplies. Some gardeners make one fertilizer that can be used with most plants. Others develop specific mixes for different kinds of plants, such as lawn grasses, vegetables, annual flowers, and trees and shrubs. Still others make mixtures for different times of the year. You can even make mixes of fast-acting, soluble natural fertilizers to use on unprepared soils or when transplanting, to ensure that plants start growing with sufficient nutrients on hand. In fact, some natural fertilizing systems rely on a starter formula for use early each season, followed later in the season by one or more slow-acting mixes.
The ingredients in the following recipes are combined by volume, not by weight. So, in addition to a bucket to keep it in, you’ll need a large scoop or cup to use for measuring. It doesn’t matter what size the scoop is, as long as you use it for all of the ingredients in the mix.
 All-Purpose Vegetable and Annual Organic Fertilizers There are many recipes for making a natural fertilizer mix for general use. These need to include nitrogen, phosphorus, potassium, and trace elements likely missing from the soil, as these are most likely the limiting nutrients.
All-Purpose Vegetable and Annual Organic Fertilizers There are many recipes for making a natural fertilizer mix for general use. These need to include nitrogen, phosphorus, potassium, and trace elements likely missing from the soil, as these are most likely the limiting nutrients.
Both of the following recipes should be adjusted in accordance with your soil test results. If the soil doesn’t need as much of a nutrient, you can leave some of the ingredient containing that nutrient out. In short, adjust the recipe in keeping with the testing laboratory’s recommendations.
In my experience, adding 0.5 inch (1.3 cm) of compost, or 6 inches (15 cm) of grass clippings when there is no compost, as a cover to these fertilizer mixes increases their efficiency in helping with microbial activity. The use of mulches always makes sense, as there are no bare soils in nature.
Grandpa Al’s Can’t Fail Recipe
4 parts fish meal or any of the nitrogen-supplying meals such as soy or cottonseed meal
1 part kelp meal
1 part rock phosphate or ¾ part bone meal
1 part dolomitic limestone, or 1 part calcitic limestone, or 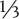 part dolomitic limestone,
part dolomitic limestone, 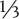 part Sul-Po-Mag, and
part Sul-Po-Mag, and 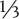 part calcitic limestone
part calcitic limestone
This mix is applied at a rate of 1 to 2 gallons per 100 square feet (3.8 to 7.6 liters per 9.3 m2) at the start of the season (that is, prior to planting), banded into the root zone. Side dress under the mulch every 4 weeks.
Nitrogen is provided by the meals. Phosphorus is provided by the rock phosphate or bone meal. Potassium is provided by the sulfate of potash and the kelp, which also supplies micronutrients along with cofactor minerals that help in enzymatic reactions.
Note that the use of limestone here is not to change the pH of the soil. It is to counter the acidic pH created by the other ingredients in the mixture. If your soils are very alkaline (and you know this because of soil tests), some lime is O.K. and needed, but you should reduce the amount of dolomitic limestone added because magnesium will tie up other nutrients at high pH.
Steve Solomon’s Recipe
3 parts cottonseed meal
1 part blood meal
1 part dolomitic or calcitic limestone
½ part bone meal
½ part kelp meal
Apply this at a rate of 6 quarts per 100 square feet (5.7 liters per 9.3 m2). Band it into the root zone for quicker results, but only if you really need them. A system that has already been natural for a few years won’t need mixing in. Side dress after removing mulch once every 3 or 4 weeks; reapply the mulch after application.
The nitrogen is supplied by the cottonseed meal and the blood meal, which supplies a much more immediate pool of nitrogen. Phosphorus comes from the bone meal, and potassium is provided by the kelp meal. As with the previous recipe, the use of limestone is not to adjust the soil pH.
 All-Purpose Vegetable and Annual Starter Solutions Again, the big negative to some gardeners in using natural fertilizers is their slow startup time. Some natural fertilizers are more soluble than others, however. This is one reason some gardeners use a liquid solution, rather than meals or powdered mixes, because nutrients that are in the water solution are immediately available.
All-Purpose Vegetable and Annual Starter Solutions Again, the big negative to some gardeners in using natural fertilizers is their slow startup time. Some natural fertilizers are more soluble than others, however. This is one reason some gardeners use a liquid solution, rather than meals or powdered mixes, because nutrients that are in the water solution are immediately available.
Quick Starter for Planting
1½ parts fish emulsion or fish powder, or ½ part bat guano
1 part liquid kelp
Dilute with water, according to the label instructions for the fish emulsion, fish powder, or bat guano. These ingredients provide a very quickly available source of nitrogen, phosphorus, and potassium. The kelp provides the trace minerals. The trick is to make sure this source of soluble nutrients gets to the root zone quickly. You can soak transplants in a diluted solution of 1 part starter to 4 parts water for a while before transplanting.
This is the only mixture that comes with warnings. First, do not use this mix in clay soils as it will deflocculate, or break apart, the clay. Second, this mixture has a lot of soluble nitrogen in it and must be mixed at the right dilution rate and then applied at the right application rate. The starting dilution rates to use are 3 tablespoons of bat guano per gallon (3.8 liters) of water, 1 teaspoon of fish powder per gallon of water, or 2 teaspoons of fish emulsion per gallon of water. Application rates are often included on the label. If not, assume that a gallon (3.8 liters) will service the plants in a 4 to 5 square foot (0.4 to 0.5 m2) garden. In fact, it is always a good idea to test the formula on sample seedlings. (Finally, you have something to do with the extra seeds in a packet.) If they don’t die after application, you have an acceptable formulation and will see them respond quickly.
 Lawn Fertilizers Lawns are a classic sink for commercial fertilizers because most chemical gardeners violate the Law of Return by removing grass clippings and raking fallen leaves instead of mulching them in. Once a gardener stops removing leaves and grass clippings, lawns need very little fertilizer input. This is especially true if clover moves in, as it hosts Rhizobia bacteria that fix nitrogen.
Lawn Fertilizers Lawns are a classic sink for commercial fertilizers because most chemical gardeners violate the Law of Return by removing grass clippings and raking fallen leaves instead of mulching them in. Once a gardener stops removing leaves and grass clippings, lawns need very little fertilizer input. This is especially true if clover moves in, as it hosts Rhizobia bacteria that fix nitrogen.
Some want a greener lawn. You can try spreading a bit of compost, 0.5 inch (1.3 cm) deep, or use Wayne Lewis’s Graceland Lawn Food, a good general-purpose lawn food tested extensively by my colleague Wayne Lewis.
Wayne Lewis’s Graceland Lawn Food
1 part soybean meal or chicken litter meal
1 part granulated molasses
A 50-pound (23-kg) bag of each, mixed, will fertilize 2000 square feet (186 m2) of lawn. Apply it after weekly watering alone no longer keeps the lawn green. Unlike commercial lawn fertilizers, you really cannot apply too much and it surely will not burn the lawn. If you are just starting out or are unsure, apply it once in the spring and see how you feel after the first month. It helps to leave grass clippings, which will provide nitrogen as per the Law of Return as well as foster microbes that will cycle the soybean and the molasses more quickly.
The soybean meal provides nitrogen and substrates for fungi, and the granulated molasses is a terrific microbial stimulant as well as a fungal food. The combination results in lawns getting ample nitrogen. If you really need to load on the nitrogen (based on soil test results), add 1 part nitrate of soda or bone meal to the recipe. These won’t last as long as the chicken litter meal or soybean meal, but you will see some quicker reactions. You’ll also have to mow more often.
These days, there are as many organic mixes on the market as there are chemical ones. Some are single-nutrient fertilizers, and others are more complete mixtures. As fertilizers, each has to comply with labeling laws and will display the N–P–K trilogy, at a minimum. Natural fertilizers add organic bulk to soils, so everything is of benefit. Chemical fertilizers don’t contain organic matter but rather fillers, which may or may not be of benefit—but are never as beneficial as organic matter.
It may be difficult to get a perfect fit between what your soil needs and what is available in a commercial mix on the shelf. Because nitrogen is the nutrient most used by plants and the one that is most often inadequate in supply (Do I hear Von Liebig’s law?), soil testing laboratories usually suggest the gardener apply an N–P–K formula that first meets the nitrogen needs of the soil.
There are lots of different ways to apply fertilizer, and each should be explored. The most prevalent is broadcasting, a technique used to spread fertilizers over large areas. Fertilizer in granular or powder form is spread over the surface by hand or by using a drop spreader. A hand-cranked broadcast spreader works on a lawn. Although fine for lawns or a large garden, broadcasting is not very efficient in some situations. The areas between rows and plants receive fertilizer, which is a waste and encourages weeds. Broadcasting also places phosphorus on the surface, where it pretty much remains unavailable to plants.
Banding, on the other hand, is a way to apply fertilizer (and compost and humus) to the soil before planting so plants will get off to a quick start. It involves putting a band of fertilizers in the root zone. This band should be about 2 inches (5 cm) away from where the plants or seeds will go in and be about 2 inches (5 cm) deeper than where seeds are planted. For transplants, place the band 3 to 4 inches (7.5 to 10 cm) below where transplants will go into the garden.
Banding is used especially when applying nutrients that are immobile in soil, particularly phosphorus and potassium, which need to be near the roots for efficient uptake. Studies have shown a 50 percent increase in phosphorus and potassium uptake when fertilizer is placed in a band, rather than broadcast.
You can spot band by putting a bit of fertilizer in a hole and planting around it. This is a great way to apply immobile phosphates and potassium in autumn, as well as some of fertilizers that take a bit of time before they are mineralized, such as colloidal phosphate and feather meal. It is also a great system for starting plants.
Garden books often tout side dressing for the second and third applications during a growing season. This entails placing the fertilizer along the row or beside a plant, usually at midseason. Place the fertilizer under mulch, or you can bury the fertilizer a bit or insert it into the top 1 or 2 inches (2.5 or 5 cm) of soil by making a slit in the mulch and soil along the side of a row or an individual plant with a knife or trowel and pouring the fertilizer in. Some gardeners use a dilute mix in water and pour on this mix as a weekly side dressing, but this probably isn’t the most efficient way to apply it due to runoff and drainage. Also, the sudden and relatively frequent applications change all sorts of conditions that affect how nutrients move to and into roots. Besides, it seems like a lot of work to make so many applications.
I’ve already mentioned what I think are the limitations of foliar spraying fertilizers. This practice is fine for a temporary quick fix for deficiencies of iron and zinc, which are mobile. The macronutrients can sometimes enter the leaves this way, too, but gardeners cannot supply plants with enough of these by foliar spraying to keep the plants thriving. The bottom line is you should not rely on foliar feeding for anything but the most mobile nutrients.

Banding organic fertilizers is a good way to put them in the root zone, where they are needed.
Once established, most perennials, trees, and shrubs don’t need much fertilizer if they are maintained in a natural system and the Law of Return is allowed to operate. The exceptions might be at planting time to ensure the plant gets nutrients while it establishes a permanent root system. If a plant is pruned often or has lots of fruit removed for consumption instead of decaying on the ground and returning nutrients to the soil, the application of fertilizer might be in order. Again, only a test will really tell. If you do apply fertilizer to trees and shrubs, apply it on or near the soil surface. In most instances, feeder roots for trees, in particular, are within the top few inches.
More and more biological organisms are being marketed to help feed plants. For example, Rhizobia inoculates for legumes are common. However, because these plant-microbe relationships are very specific, you should do some research to ensure a match before wasting time and money buying them. Rather than purchasing these bacteria, you can gather some root material and open up the nodules that are created. The pink stuff is coated with Rhizobia. Roll seeds in it or mix it into soil.
Mycorrhizal fungi mixes are also available commercially. Fungal spores and propagules only germinate when they receive root exudates, so contact with roots helps. Similarly, rolling legume seed or roots into Rhizobia mix will help infect the legume plant when the seed germinates.
Many mycorrhizal fungi are ubiquitous in established natural systems. Therefore, they usually only need to be introduced in new systems, when annuals and vegetables are started indoors, and when plants are grown in compost, which doesn’t have much by way of mycorrhizal spores. There is the question of specificity of fungi to host plant, but mycorrhizal mixes usually contain enough different species to infect some roots. It’s not quite as easy with Rhizobia because of a higher specificity, but if you have a legume such as clover or soybeans or peanuts, you can cut open nodules and use the contents on similar legumes.
You can also go out and collect local mycorrhizal fungi and spores. You can’t see the endomycorrhizal fungi without a microscope and staining them, and it takes a trained eye and a good magnifying glass to find ectomycorrhizal fungi (unless they are fruiting, in which case their mushrooms are a great source of spores). However, by taking soil from root areas of thriving plants you will surely get spores, and you can inoculate the soil of the new plants by adding some of this soil to their mix near or on their roots
Soil testing must become part of the thinking gardener’s activities. We have the knowledge to follow how nutrients move to plants and get into them and a rudimentary understanding of what they do inside. However, we can’t see them in the soil or in the plant. Only by testing soils can the gardener know what needs to be adjusted to ensure the presence of all the essential nutrients in adequate quantities.
You have the information to make educated choices, rather than just blindly following some label on a box because it makes you (and some ad agency) feel good. Now that you know about the botany, cellular biology, soil biology, and chemistry involved in how plants eat, consider yourself their gourmet chef. No more generic fast foods that ruin the soil food web, change the pH, introduce competing nutrients, or chemically tie them up. After a soil test, of course, and based on the resulting recommendations, you can prepare meals that are perfectly suited to your plants.
 Use natural fertilizers because they feed the soil food web that creates soil structure and cycles nutrients to plants.
Use natural fertilizers because they feed the soil food web that creates soil structure and cycles nutrients to plants.
 Natural fertilizers are derived from plant and animal by-products as well as rock.
Natural fertilizers are derived from plant and animal by-products as well as rock.
 Nutrients in all but the most soluble natural fertilizers are only available when they are mineralized by the soil food web.
Nutrients in all but the most soluble natural fertilizers are only available when they are mineralized by the soil food web.
 Good natural sources of nitrogen include alfalfa meal, bat guano, blood meal, cottonseed meal, corn gluten meal, feather meal, fish emulsion, fish meal, fish powder, hydrolyzed fish, human hair, human urine, soybean meal, and Chilean nitrate.
Good natural sources of nitrogen include alfalfa meal, bat guano, blood meal, cottonseed meal, corn gluten meal, feather meal, fish emulsion, fish meal, fish powder, hydrolyzed fish, human hair, human urine, soybean meal, and Chilean nitrate.
 Phosphorus is provided by animal bone meal, bat guano, colloidal rock phosphates, and crab shell meal.
Phosphorus is provided by animal bone meal, bat guano, colloidal rock phosphates, and crab shell meal.
 Greensand, wood ashes, and sulfate of potash are good sources of potassium.
Greensand, wood ashes, and sulfate of potash are good sources of potassium.
 Calcitic limestone and dolomitic limestone are commonly applied for calcium deficiencies and to adjust the soil pH.
Calcitic limestone and dolomitic limestone are commonly applied for calcium deficiencies and to adjust the soil pH.
 Good natural sources of trace elements include shrimp shell meal, kelp meal, kelp powder, and liquid kelp.
Good natural sources of trace elements include shrimp shell meal, kelp meal, kelp powder, and liquid kelp.
 Biofertilizers include symbiotic, nitrogenfixing Rhizobia and Frankia; free-living, nitrogen-fixing Azotobacter and Azospirillum; and phosphate-solubilizing bacteria. Some fungi increase the movement of phosphorus to roots, and mycorrhizal fungi deliver phosphorus, copper, zinc, molybdenum, and nitrogen to plant roots.
Biofertilizers include symbiotic, nitrogenfixing Rhizobia and Frankia; free-living, nitrogen-fixing Azotobacter and Azospirillum; and phosphate-solubilizing bacteria. Some fungi increase the movement of phosphorus to roots, and mycorrhizal fungi deliver phosphorus, copper, zinc, molybdenum, and nitrogen to plant roots.
 Growth-promoting rhizobacteria form symbiotic relationships with plants that aid in the synthesis of nitrogen.
Growth-promoting rhizobacteria form symbiotic relationships with plants that aid in the synthesis of nitrogen.
 Earthworm castings concentrate nutrients and create humus.
Earthworm castings concentrate nutrients and create humus.
 You can make your own fertilizer mixes with the recipes included in this chapter.
You can make your own fertilizer mixes with the recipes included in this chapter.
 The nutrient amounts in commercial natural fertilizers may not meet your soil’s exact needs. In that case, buy according to your soil’s nitrogen needs.
The nutrient amounts in commercial natural fertilizers may not meet your soil’s exact needs. In that case, buy according to your soil’s nitrogen needs.
 Apply fertilizer by broadcasting, banding below the root zone, or side dressing, depending on needs.
Apply fertilizer by broadcasting, banding below the root zone, or side dressing, depending on needs.
 Banding is especially useful for phosphorus and potassium.
Banding is especially useful for phosphorus and potassium.
 Mycorrhizal fungi and Rhizobia can be purchased or collected locally and applied to the roots.
Mycorrhizal fungi and Rhizobia can be purchased or collected locally and applied to the roots.