

 Each nutrient is an element, a substance composed of identical atoms.
Each nutrient is an element, a substance composed of identical atoms.
 An atom has positively charged protons in its nucleus, which is surrounded by a cloud of negatively charged electrons.
An atom has positively charged protons in its nucleus, which is surrounded by a cloud of negatively charged electrons.
 When atoms of the same or different elements share electrons, a chemical bond is formed.
When atoms of the same or different elements share electrons, a chemical bond is formed.
 Nutrients form three types of bonds: covalent, ionic, and hydrogen bonds. These affect the qualities and availability of the various nutrients.
Nutrients form three types of bonds: covalent, ionic, and hydrogen bonds. These affect the qualities and availability of the various nutrients.
 Plants take in the nutrients in order to build the four types of molecules that are necessary for life: carbohydrates, proteins, lipids, and nucleic acids.
Plants take in the nutrients in order to build the four types of molecules that are necessary for life: carbohydrates, proteins, lipids, and nucleic acids.
 Adenosine triphosphate (ATP) serves as the energy currency in cells.
Adenosine triphosphate (ATP) serves as the energy currency in cells.
 Enzymes are proteins that dramatically speed up the millions of chemical reactions that occur in plant cells.
Enzymes are proteins that dramatically speed up the millions of chemical reactions that occur in plant cells.
 Osmosis and diffusion are two processes that allow water and nutrients to move across membranes without the input of energy.
Osmosis and diffusion are two processes that allow water and nutrients to move across membranes without the input of energy.
 The active transport of nutrients across a cell membrane requires energy.
The active transport of nutrients across a cell membrane requires energy.
CHEMISTRY DEALS with atoms and molecules and how they react with other atoms and molecules. That’s all chemistry is. These behave very predictably. Yes, it is chemistry, and many people have a fear of it. Just take a deep breath or two and dive in. There won’t be any tests or grades. You don’t have to memorize. “You will learn,” as my mother used to incorrectly say, “by osmosis.” Pretty soon you will have enough familiarity with the necessary elements. Don’t let the chemist’s vocabulary get in the way of learning.
All substances are made up of atoms. An atom is the smallest unit of an element that retains the properties of that element. Atoms consist of a central nucleus that has a certain number of protons, which are positively charged particles, and neutrons, which have no charge, depending on which of the 118 elements the atom belongs to. The atom’s nucleus is surrounded by lighter particles known as electrons. These carry a negative electrical charge.
Atoms are often depicted as planets with circling moons, with the planet representing the nucleus comprising protons and neutrons and the moons representing the circling electrons. The orbiting planet view of an atom is an oversimplification of atomic structure (and I am all about simplification). The electrons actually form a swirling cloud or shell around the entire nucleus, but for our purposes the planetary model suffices.
There is an electromagnetic force between the positively charged protons in the nucleus and the negatively charged electrons surrounding it that keeps the electrons in orbit. An atom can have several orbits of electrons, with each consecutive orbit having a higher energy level.
By definition, atoms are small, a whole new level of smallness, in fact. If an orange were the size of the Earth, each atom in it would be the size of an orange. Or imagine that a pea represents the size of an atom. The cell we just studied would be the size of an ocean-going cargo vessel. Just remember, as small as it is, an atom still has three dimensions.
Each element has a unique number of protons, its atomic number. For example, hydrogen has one proton in its nucleus, and nitrogen has seven. But for the most part, and certainly for our purposes, it is the number of electrons in the outer orbit of an atom that determines its chemical behavior.
Electrons like to pair up whenever possible. Most of the chemical behavior that gardeners care about is a result of pairing up all of the electrons in the outer shell of an atom. This is the driving force of chemistry and, indeed, life on an atomic level. (Indeed, many would argue, it’s the driving force of life on any level.) The outer shell of most atoms can hold four pairs of electrons, or eight electrons in total. This is called an octet, and Lewis diagrams are used to show the electron configuration around particular atoms and combinations of atoms in pairs or as loners.
The outer orbit of electrons of an atom is known as the valence orbit. Sometimes there are less than all eight electrons in the shell, but there are never more than eight. Because electrons tend to form pairs, an atom with fewer than four pairs in its valence orbit is highly reactive. In a sense, an atom seeks out the electrons of other atoms in order to pair off the electrons in its outer orbit.
Chemists use shorthand to represent elements and the combinations they make. Thus, water is H2O. This means that each water molecule consists of two hydrogen (H) atoms and one oxygen (O) atom. It is the completion of the pairing of electrons in the valence orbits of the hydrogen and oxygen atoms that results in chemical bonding of the three and the creation of water.
Because valence electrons like to share their orbits, single atoms rarely occur. Instead, when vacancies appear, other atoms come to complete the pair. This causes the atoms to bind together to form molecules. A molecule is the smallest unit of a compound substance that retains the properties of that substance. Just as an atom can’t be further divided, if you tried to divide a molecule of a compound, you would end up with its constituent atoms. If the atoms are the same, the molecule is an elemental one (such as nitrogen, N2, which is composed of two nitrogen atoms bonded together). If they are different, the combination results in formation of a compound (such as water, H2O). In every case, however, it is the electrons in the outer shell that do the bonding and create the chemistry.
Electrons can behave in different ways, which results in different kinds of bonds, each with special characteristics that give the molecules different properties. There are just three kinds of bonds of importance: covalent, ionic, and hydrogen bonds.
A covalent bond is formed by an electron (or electrons) in one atom’s outer shell that also spends time orbiting in a second atom’s outer shell in order to complete an empty pair. This creates a force that holds the two atoms together: a covalent force.
Covalent bonds result in molecules that have very tightly bound atoms. These are very stable, meaning that they are not usually attracted to other molecules. The ultimate strength of covalent bonds depends on the number of unfilled pairs that are completed—that is, the number of bonds. Sometimes the bond involves the sharing of only one or two electrons between two atoms; in fewer instances, three electrons are shared. Because more bonds means there is a stronger attraction between the atoms, triple covalent bonds are really, really strong.
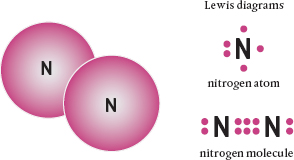
A nitrogen molecule is composed of two nitrogen (N) atoms. As the Lewis diagrams show, when bound together each atom has an octet of electrons in its outer shell, forming a triple covalent bond.
The number of electrons available to complete pairs allows chemists to predict chemical behavior. First, they can predict how strong bonds will be. In addition, they can predict how many bonds there are between atoms. (We won’t, but chemists use Lewis diagrams to figure this out and make compounds.) The atoms of organic molecules—those made up of carbon and hydrogen and often with the addition of oxygen and nitrogen—are joined by covalent bonds.
There are variations of covalent bonding. A nonpolar covalent bond is one in which the shared electron(s) spend equal time in the orbit of each atom, so that each part of the molecule has the same charge. Because the charge is uniform across the molecule, nonpolar molecules are not attracted to other molecules. Atoms of the same element always bond together by forming nonpolar covalent bonds.
Atoms of different elements, however, have different abilities to share. This results in some interesting behaviors and a second type of covalent bond, the polar covalent bond. Electrons of different elements spend more time circling one atom than the other. The atom that holds the electron longest becomes slightly more negatively charged, whereas the other atom becomes slightly more positive. The molecule ends up with opposite charges at different locations. These locations are called poles. The most famous of the polar molecules is the water molecule, which has a negative charge on the oxygen atom and positive charges on the hydrogen atoms. This is important because on a molecular or atomic level, like charges repel each other and opposite charges attract.
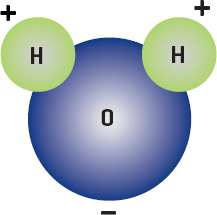
A water molecule is made up of two hydrogen atoms and one oxygen atom. Water is a polar molecule, as it has two different charges on it.
Most atoms are electrically neutral because they contain the same number of orbiting electrons as they have protons in the nucleus. However, for all sorts of reasons, individual atoms can gain or lose electrons and thus develop a charge. A molecule with a charge is an ion. Cations are positively charged ions, and anions are negatively charged ions. Ions are very reactive, meaning they end up combining with other reactive molecules. The mutual attraction of oppositely charged ions results in ionic bonding.
A subscript number in a chemical formula indicates the number of atoms of that element. The presence of a superscript plus (+) or minus (−) sign in a chemical formula indicates that an ion is a cation or an anion, respectively. The number after the charge gives an indication of the magnitude of the charge. Thus, an ion of iron is symbolized by Fe2+2, meaning it has two atoms of iron (Fe) and two positive charges.
The diameter of an ion’s outer orbit determines its size. Hydrogen cations (H+) are the smallest ions because they don’t have any electrons at all. The positive charge comes from the lone proton in the nucleus. For this reason, hydrogen ions are often referred to as protons.
The hydrogen bond is a very special bond, the one that gives water molecules their unusual ability to stick together. Because water is so vital to life, hydrogen bonds have been extensively studied, and discoveries about them are still being made. The field is bewildering, but it all can be distilled for our purposes.
A hydrogen bond forms between a hydrogen atom and a negatively charged atom that is itself part of a molecule or a group of atoms. What is important is that a hydrogen atom can be attracted to two atoms at once instead of just one. The bond usually occurs between hydrogen and oxygen, nitrogen, or fluorine. Hydrogen bonds can form between two molecules or within an individual molecule. They are generally pretty weak, estimated to be about 5 percent the strength of a covalent bond. However, when there are lots of hydrogen bonds, molecules can bond together strongly. This is a great example of strength in numbers.
Most important for us is that water molecules are held together by hydrogen bonds. Water is a polar covalent molecule. The electron sharing arrangement in the molecule results in the oxygen end of the molecule having a higher negative charge, whereas the two hydrogen ends are more positive. When water molecules are close enough, the positively charged hydrogen atom ends form hydrogen bonds with the negatively charged oxygen atoms of other water molecules. So, each water molecule is hydrogen bonded to four other water molecules (and so forth). This is enough to hold them together tightly. Although individual hydrogen bonds are the weakest of the three bonds, when there are lots of them, it takes quite a bit of energy to break them all. Although they break, hydrogen bonds reform continually. This is why water stays in a drop instead of behaving like a powder.
Hydrogen bonds affect the way a molecule is shaped. (Remember, we are thinking in three dimensions.) They cause binding molecules to attach at an angle. It is the presence of hydrogen bonds that results in the famous double-helix shape of the DNA molecule. Hydrogen bonds are also the reason proteins have their various twisted and folded shapes, which make them so functional and important to life processes.
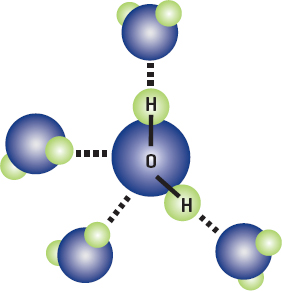
Water molecules are bonded to each other by hydrogen bonds (dotted lines).
Without getting into complicated chemistry (at this point, at least), hydrogen bonds are why water can exist as a gas, solid, and liquid at the same temperature, and the reason water can be taken up into a sponge or a tall redwood. Hydrogen bonds are why there are smaller temperature extremes near large bodies of water, and why water can remain in a vapor state for so long. All of these have to do with the impact of hydrogen bonds on melting and boiling points, solubility, and viscosity.
Hydrogen bonds explain why water is a great solvent. A solvent is a substance into which other molecules dissolve. Water can dissolve more elements than any other liquid. Charged molecules entering water are surrounded by water molecules and attach to either a positive oxygen atom or a negative hydrogen, depending on their charge. However, hydrogen bonds also hold the water molecule to other water molecules. Thus, the solutes become dissolved in water. In fact, water is such a good solvent that it is extremely difficult to find it in a truly pure state.
Note that in order to be soluble in water, a substance must also be polar or otherwise charged. This is important to how plants take up nutrients because the nutrients are dissolved in water. This means that plant nutrients have to be charged. In almost all instances, nutrients enter plants as ions, some cations and some anions.
Chemists use the terms acid and base often. Gardeners do not. Again, let’s put some of our new knowledge to use. Acids are substances that produce hydrogen ions (H+) in water, whereas bases produce hydroxyl ions (OH−) in water. Even simpler, an acid is any substance that will react with a base.
Acids and bases are ions. When they react, they form salts. Salts are simply the end products of what is known as a neutralization reaction, two ions neutralizing each other. Salts do not have an electrical charge because the ions that make them neutralize each other.
When put into water, however, salts dissolve and in doing so they split or hydrolyze the water molecule. Salts can create an excess of hydroxyl ions (OH−) or hydrogen ions (H+). A liquid’s pH is the measure of these ions, so if the salt produces OH−, it is a basic salt, and if it produces H+, an acid salt. Many fertilizers are salts. It’s a good idea to be able to tell whether things you are putting in the soil are going to make it more acidic or more basic.
Amazingly, plants are simply the combination of four groups of molecules. In fact, so is all life. The only difference between a gardener and a bacterium is the way carbohydrate, lipid, protein, and nucleic acid molecules are mixed.
Carbohydrates are carbon-based molecules made up of combinations of carbon, oxygen, and hydrogen, and for the simpler ones there is usually one water molecule for each carbon atom. The word carbohydrate means “carbon water.”
Sugars, starches, and cellulose are all carbohydrates. The only difference between them is how many carbon-water molecules they contain. Really simple carbohydrates, monosaccharides (or simple sugars), can have up to six carbons (and that means they can have up to twelve water molecules). Glucose (C6H12O6) is the most common monosaccharide in plants and animals.
Glucose is used for energy storage as well as a base for adding on other elements to make lots of other different kinds of molecules. How common is it? The world’s plants use more than 100 billion tons of carbon, hydrogen, and oxygen taken from the atmosphere to produce enough glucose molecules through photosynthesis to fill a line of tanker trucks 25 to 30 million miles long.
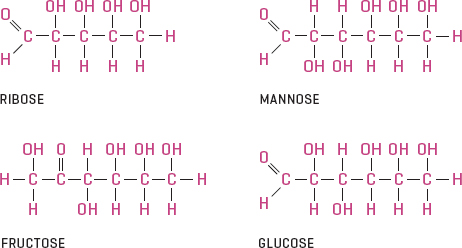
Connecting these simple sugar monomers in various combinations results in more complex and different sugar molecules, known as polysaccharides.
If you connect two monosaccharides, a disaccharide is formed. Sucrose (C12H22O11) is a disaccharide. This is the usual form of sugar when it is transported in a plant. (Pass the maple sucrose, please.) Similarly, if lots of monosaccharides (or disaccharides, for that matter) are linked, they form polysaccharides. Starch (C6H10O5)n, the preferred form of sugar for storage in plants, is a polysaccharide. So is cellulose, the material that makes up the outer walls of plant cells. The difference between these two carbohydrates is how the glucose molecules are lined up and linked together. Polysaccharides don’t readily dissolve in water.
Lipids include fats, oils, and waxes. The base of these molecules is hydrogen and carbon and a little bit of oxygen. Lipids are nonpolar (no charge, no attraction) and therefore don’t dissolve in water. They are hydrophobic, meaning they are pushed together when exposed to water. This is why waxes can block the flow of water. They coagulate in water. It is why an oil-and-vinegar salad dressing has to be shaken to get a semblance of a mixture.
Steroids are also lipids. These molecules consist of four carbon rings to which are attached various molecules that determine their function. Of course, there are the phospholipids, which form all those important cellular membranes that contain transport proteins, and they prevent or delay the entry of unwanted molecules into the cell.
Proteins are nitrogen-based molecules. They are composed of amino acids, each of which has an amino group (NH2) and a carboxyl group (COOH), plus a side-chain molecule whose composition can vary. (Whew.) The take-home picture is that proteins are large, complicated molecules. They have a lot of bonds that cause them to fold, twist, and change shapes. In fact, proteins are usually folded and the manipulation of these folds results in some of their properties.
Proteins are made up of smaller units, amino acids. Some of these are hydrophilic, and some are hydrophobic. When a protein is exposed to water (and what isn’t in life?), it will fold itself up so that the hydrophilic amino acids are exposed to water while protecting the hydrophobic amino acids from it.
Proteins are used to store and move materials, such as membrane transport proteins. Nothing gets through these nitrogen-based molecules unless it’s supposed to get through. They can signal to cause the start, end, or change in activities. Proteins create structure and serve as catalysts to speed up a cell’s chemical reactions. They are vitally important to every aspect of life.
Nucleic acids, DNA and RNA, are special molecules comprising carbon, hydrogen, oxygen, phosphorus, and nitrogen. These are also very large molecules. DNA and RNA molecules hold the genetic code, the instructions that are used to build cells and everything in them. Among the most important role for nucleic acids is the duplication of proteins, which, despite their numbers and importance, cannot duplicate themselves.
Each nucleic acid is made up of smaller units consisting of a sugar, a phosphate group, and one of two kinds of nitrogen bases. These are nucleotides. Only five nucleotides (adenine, thymine, cytosine, guanine, and uracil) combine in various combinations to make all the DNA and RNA that code life. It is nucleotides, along with hydrogen bonds, that create the famous double helix of DNA.
That is all it takes to build a plant: carbohydrates, lipids, proteins, and nucleic acids. These four primary compounds exist in all living things. All of these organic compounds (molecules containing carbon and hydrogen) fit into one of these four categories, and each and every one of these molecules is composed of the essential nutrients.
The terms oxidation and reduction are two prime examples of chemists making things more complicated than they need to be. Both are processes that are important to cellular metabolism and occur all the time. In fact, when one occurs, so does the other.
Reduction is simply the addition of electrons, which turns out to be extremely important to cellular metabolism. So is oxidation, which is just the opposite, the loss of electrons. (When scientists say glucose undergoes oxidation during respiration, all they mean is that sugar loses electrons.) Oxidation and reduction occur simultaneously. As the electron is transferred from one molecule to another, one is oxidized and the other is reduced.
Plants require energy. One molecule in particular, adenosine triphosphate (ATP), serves as the currency of energy in plant cells. ATP, as its name suggests, contains three phosphate ions (PO4−3). One is attached to a carbon ring that is attached, in turn, to a nitrogen-based molecule, and the other two phosphates are bonded to it. This results in a line of phosphates with two phosphate-to-phosphate bonds.
If a cellular function requires energy, one of the phosphates bonds of ATP is broken to remove a phosphate molecule, thus forming adenine diphosphate (ADP). The energy that held the two phosphates together is released. Similarly, creating phosphate bonds (that is, adding a phosphate to ADP to make ATP) serves as a way of transferring energy for storage.
Phosphorylation is the name given to the transfer of phosphate groups from one molecule to another molecule to release energy. Photosynthesis is all about phosphorylation, as is respiration, which uses the sugar made during photosynthesis. Photosynthesis breaks ATP bonds to make sugar, and respiration uses the sugars to make ATP.
All of the energy used in cells is generated in chloroplasts and mitochondria. In the chloroplasts, chlorophyll captures light energy and uses most of it to convert water (H2O) and carbon dioxide (CO2) into glucose (C6H12O6). Some of this light energy is stored as ATP. Most of the cellular ATP, however, is produced in mitochondria, where glucose is oxidized to synthesize ATP.
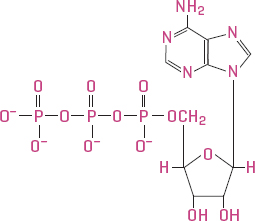
Adenosine triphosphate (ATP) is the universal currency of energy in biological systems. The bonds between the phosphates contain the energy used by plant cells.
The question arises as to why plants need chloroplasts to make sugar for energy if they can make ATP directly in mitochondria. Part of the answer is that redundancy in key operations is important, but the sugar has other uses as well. We usually don’t think of it as such, especially given sugar’s bad rap regarding accumulation of both fats and cavities, but plain old glucose is a basic building material for everything in a cell. Its carbon, hydrogen, and oxygen atoms are used to make all of those nucleotides, lipids, and proteins.
In any case, ATP is the energy currency of life. The energy is used to link together molecules and chains of molecules during synthesis. The energy from ATP can make proteins change shape, which serves lots of functions. ATP’s energy can break apart water, and it is used to recombine carbon, hydrogen, and all of the nutrients a plant needs.
Catalysts are substances that increase or decrease the rate of a chemical reaction. Enzymes are proteins that act as catalysts. They work in several ways.
First, enzymes can form a template that holds two or more molecules (substrates) in just the position so that they bind or unbind. Second, an enzyme can get between the atoms of a molecule (or molecules) and force them into a shape that results in bonding or other reaction. Finally, enzymes can change electrical charges on substrates by taking away electrons (oxidation) or adding them (reduction).
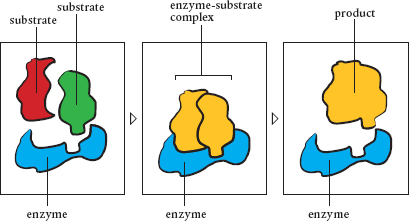
An enzyme in action, connecting two substrates and making a new molecule
The increased speed afforded by the presence of enzymes is nothing short of phenomenal. Reactions occur millions of times faster, which means nothing is left to chance. It’s a good thing these speeds can be achieved in biological systems. Cellular synthesis, decomposition, and a host of other critical activities such as respiration and photosynthesis would simply be to slow to sustain life if it were not for enzymes.
Some enzymes are useless without the presence and involvement of a cofactor, a non-protein molecule that must bond to an enzyme before there is any catalytic activity. The cofactor can’t speed things up on its own. Both are needed, which makes cofactors pretty important.
There are different kinds of cofactors, depending on how strong the bond is with the protein enzyme. They can be inorganic or organic, in which case they are known as coenzymes. Many cofactors and coenzymes are vitamins or made from vitamins, and some are metal ions. A metal is an element that conducts both heat and electricity. Several of a plant’s trace nutritional elements are metal ions, such as copper, zinc, and iron, which are needed to assist all the catalytic activity in plant cells. They extend the range of enzymes, allowing the same ones to work in different ways.
Diffusion is the movement of atoms, ions, or molecules from areas of high concentration to areas of lower concentration. This movement is passive—that is, it does not require the input of energy to the system. For example, if you open a container of really smelly fish fertilizer, the smell soon diffuses throughout the room. Molecules carrying the fishy smell spread out of the bottle, where they are highly concentrated and constantly bumping into each other, and move out into the room, where the concentration is lower. Diffusion normally occurs faster at higher temperatures.
Concentration gradient is the term applied to the difference in concentrations. If everything else is equal, similar molecules always move toward the area of least concentration, or down the concentration gradient, without the application of additional energy. This process is called passive transport.
Osmosis occurs when water is the diffusing agent. If you have pure water on one side of a semi-permeable membrane and water with a solute in it on the other side, the pure water will flow through the membrane to the water that contains the solutes because there is a lower concentration of water molecules in the solution. The solutes, if they can get through the membrane, move in the opposite direction. In this case, it is diffusion, not osmosis.
In plant roots, molecules of water, oxygen, ammonium, and carbon dioxide are small enough—or in the case of water have the right chemistry—to move into plant roots via diffusion though the plasmalemma without the input of energy. Some nutrients diffuse into the plant, but instead of diffusing through the membrane, they travel through the proteins embedded in the membrane. These proteins help the nutrients through by changing shapes or utilizing an internal electrical charge, for example, and this is considered facilitated diffusion because it is still diffusion but helped by a protein.
Root cells can buck the rules and get a nutrient molecule to move against its natural concentration gradient. This is known as active transport, and it requires the input of energy. There are several ways to add energy to transport materials against their concentration gradient. Ion pumps, small molecular motors, add energy that can be used directly or indirectly to move ions across a membrane through a transport protein.
A carbon atom can make four bonds with other atoms, ions, or molecules. Carbon serves as the backbone of all of the organic molecules necessary for life.
Let’s consider the implications of being able to make four bonds by starting with atoms that can only make two. Such atoms can only be linked together to form long chains. They can only make linear structures. When an atom has the ability to make three bonds, things take a different shape. Attachments can be made that form three angles and three ends on which to make chains and an entirely different shape. You can see where this is going.
A carbohydrate is an example of an organic molecule. Technically, organic molecules are those that contain carbon and hydrogen, although some definitions include oxygen as well.
When an atom can link to four items, there can be a center with four distinct chains, allowing for much larger, more complex shapes. The backbone of any organic molecule is a carbon atom and its four bonds. The side chains, up to four, give the particular carbon molecule its unique chemical characteristics.
That should be enough chemistry to easily follow the rest of the story. Again, don’t get hung up memorizing here. Things will be repeated, and you can always come back for reference. If new chemical concepts are needed to flesh things out, they will be explained as we go.
The key things to remember are that chemistry and chemical reactions are all about bonds, and bonds are all about electron orbits, missing electrons, sharing electrons, and transferring electrons.
 Atoms are the smallest unit of an element. An atom’s nucleus contains protons and neutrons. The negatively charged electrons orbit around the nucleus.
Atoms are the smallest unit of an element. An atom’s nucleus contains protons and neutrons. The negatively charged electrons orbit around the nucleus.
 Molecules are the smallest unit of a compound. They result from atoms bonding together.
Molecules are the smallest unit of a compound. They result from atoms bonding together.
 A covalent bond is formed when two atoms share electrons in their outer shells. Molecules formed by covalent bonds are very stable.
A covalent bond is formed when two atoms share electrons in their outer shells. Molecules formed by covalent bonds are very stable.
 Ionic bonds are formed when ions with opposite charges (that is, positively charged cations and negatively charged anions) are attracted to one another.
Ionic bonds are formed when ions with opposite charges (that is, positively charged cations and negatively charged anions) are attracted to one another.
 A hydrogen bond forms between a hydrogen atom and a negatively charged atom that is part of a molecule or a group of atoms. These are generally fairly weak bonds, but they can make up for this weakness by occurring in large numbers.
A hydrogen bond forms between a hydrogen atom and a negatively charged atom that is part of a molecule or a group of atoms. These are generally fairly weak bonds, but they can make up for this weakness by occurring in large numbers.
 When put into water, salts dissolve and split some of the water molecules. Salts that create hydroxyl ions (OH−) are basic salts, and those that produce hydrogen ions (H+) are acid salts.
When put into water, salts dissolve and split some of the water molecules. Salts that create hydroxyl ions (OH−) are basic salts, and those that produce hydrogen ions (H+) are acid salts.
 Carbohydrates consist of various combinations of carbon, oxygen, and hydrogen. Sugars, starches, and cellulose are carbohydrates.
Carbohydrates consist of various combinations of carbon, oxygen, and hydrogen. Sugars, starches, and cellulose are carbohydrates.
 Lipid molecules have no charge and therefore don’t dissolve in water. Fats, oils, and waxes are examples of lipids.
Lipid molecules have no charge and therefore don’t dissolve in water. Fats, oils, and waxes are examples of lipids.
 Proteins are large, complicated molecules made up of amino acids. They have a lot of bonds that cause them to fold, twist, and change shape.
Proteins are large, complicated molecules made up of amino acids. They have a lot of bonds that cause them to fold, twist, and change shape.
 Nucleic acids (DNA and RNA) are very large molecules comprising carbon, hydrogen, oxygen, phosphorus, and nitrogen. These molecules hold the genetic code.
Nucleic acids (DNA and RNA) are very large molecules comprising carbon, hydrogen, oxygen, phosphorus, and nitrogen. These molecules hold the genetic code.
 Oxidation is the loss of electrons, and reduction is the addition of electrons.
Oxidation is the loss of electrons, and reduction is the addition of electrons.
 Adenosine triphosphate is the universal currency of energy in biological systems. When bonds between the phosphates in these molecules are broken, energy is released.
Adenosine triphosphate is the universal currency of energy in biological systems. When bonds between the phosphates in these molecules are broken, energy is released.
 Enzymes are proteins that greatly speed up chemical reactions, making them millions of times faster.
Enzymes are proteins that greatly speed up chemical reactions, making them millions of times faster.
 Cofactors and coenzymes are molecules that assist enzymes. Vitamins and metal ions are some examples of these.
Cofactors and coenzymes are molecules that assist enzymes. Vitamins and metal ions are some examples of these.
 Diffusion is the movement of atoms, ions, or molecules from areas of high concentration to areas of lower concentration. The movement of water along a concentration gradient is known as osmosis. Both diffusion and osmosis are examples of passive transport.
Diffusion is the movement of atoms, ions, or molecules from areas of high concentration to areas of lower concentration. The movement of water along a concentration gradient is known as osmosis. Both diffusion and osmosis are examples of passive transport.
 Active transport occurs when atoms, ions, or molecules are moved against a concentration gradient, which requires the input of energy.
Active transport occurs when atoms, ions, or molecules are moved against a concentration gradient, which requires the input of energy.
 The backbone of an organic molecule is a carbon atom and its four bonds.
The backbone of an organic molecule is a carbon atom and its four bonds.