

 Carbohydrates, such as the celluloses that make up cell walls, are built from smaller sugar molecules produced via photosynthesis.
Carbohydrates, such as the celluloses that make up cell walls, are built from smaller sugar molecules produced via photosynthesis.
 Proteins are constructed from various combinations of the twenty amino acids, which are nitrogen-based molecules.
Proteins are constructed from various combinations of the twenty amino acids, which are nitrogen-based molecules.
 Specialized proteins are involved in moving molecules and nutrient ions across cell membranes.
Specialized proteins are involved in moving molecules and nutrient ions across cell membranes.
 Enzymes are specialized proteins that are necessary to catalyze chemical reactions that occur in plant cells.
Enzymes are specialized proteins that are necessary to catalyze chemical reactions that occur in plant cells.
 The building blocks of lipids are fatty acids and glycerol.
The building blocks of lipids are fatty acids and glycerol.
 Lipids, which do not dissolve in water, are important components in membranes and other barriers that regulate what can enter a plant or a plant cell.
Lipids, which do not dissolve in water, are important components in membranes and other barriers that regulate what can enter a plant or a plant cell.
 DNA and RNA, two important nucleic acids, are huge molecules that hold and replicate the genetic code, which controls the chemical reactions used to make all of the compounds in a plant.
DNA and RNA, two important nucleic acids, are huge molecules that hold and replicate the genetic code, which controls the chemical reactions used to make all of the compounds in a plant.
WHAT HAPPENS to all of the essential nutrients after they enter the plant? Because plants are autotrophic, these nutrient ions are used by the plant to make all of the molecules necessary for the plant to grow, to sustain and maintain itself, and to regenerate. All of this work is done in individual plant cells.
About 80 percent of the molecules in a plant are imported ions and water molecules. The remaining 20 percent of molecules are made in the plant using those ions and water. These new synthesized molecules are what make gardening such a fantastic hobby.
In the chemistry chapter we touched on the molecules of life, the term scientists use to describe these synthesized molecules. Again, there are four main groups: carbohydrates, proteins, lipids, and nucleic acids. With these, plant cells can make everything needed for growth and reproduction.
Let’s do some imagination stretching. First, let’s consider how many theoretical permutations a plant can make with the seventeen essential nutrients. The answer is 35,568,742,896,000, which is a truly mind-blowing number of possible molecules. Obviously, not all of these combinations are possible because of electron bonding restrictions, lack of electron pairs, molecular shape due to special bonds, and other topics we have covered. Still, a plant cell has 100 to 200 trillion atoms, so we know there is a huge number of compounds in a plant cell. And there needs to be. A DNA strand can have hundreds of thousands to millions of nucleotide pairs.
It does take imagination to acknowledge that these diminutive plant cells contain lots of molecules of all sizes and shapes. It is much more difficult to comprehend that these molecules are being made by the trillions every single second in any plant you see. Molecular bonds are what make the combination of atoms into molecules possible.
Most people are familiar with carbohydrates for dietary reasons at least. These molecules are produced by plants using photosynthesis. Carbohydrates consist of carbon, oxygen, and hydrogen atoms and usually have a base formula of C(H2O)n—that is, a combination of carbon and water molecules. In plant cells, sugars and starches are the key synthesized carbohydrates.
Precisely how carbohydrates are linked results in groupings called monomers, dimers, or polymers, which are single-, double-, or multi-chained molecules, respectively. Glucose (C6H12O6) is a monomer and is what plant and animal cells break down to release energy. If you change the shape of glucose by rearranging some of its bonds, it becomes fructose (also C6H12O6). Many carbohydrates names end in -ose.
Two monomers link together to form a dimer. Linking two glucose molecules produces the disaccharide maltose (C12H22O11), and a fructose and a glucose come together to make sucrose (also C12H22O11).
Combining three or more monomers or one monomer and a dimer results in a multi-chain polysaccharide, such as starch ([C6H10O5]n, where n indicates the number of repetitions of this subunit), which stores energy in plant roots. In fact, starch is vital for energy in both plants and animals. Carbohydrates have lots of hydrogen bonds, which is one reason why they are such good energy and storage molecules. Plants capture the energy from sunlight, and then store that energy in the form of carbohydrate molecules.
Cellulose contains half of all the organic carbon in the Earth’s biosphere. This polysaccharide carbohydrate (also [C6H10O5]n) is found in all plant cell walls. The n for cellulose can be 500 to 5000 subunits long. As noted, these carbohydrate molecules play a crucial structural and protective role for the plant.
Protein molecules are also made up of carbon, oxygen, and hydrogen atoms plus a fourth element, nitrogen. Amino acids are the smallest parts of protein molecules, the monomers that make up proteins. Twenty amino acids exist in nature.
Each amino acid has the same basic structure: a central carbon atom to which is attached a hydrogen atom (H), an amino group (NH2), and a carboxyl group (COOH), which is what makes these molecules acids. The remaining carbon bond (there are four, remember) links with a side chain group that gives the amino acid its uniqueness. With just twenty amino acids to work with, a plant cell can form long and short chains and lots of combinations to make a whole lot of different kinds of proteins—in fact, any protein needed by them, you, me, and almost everything else on Earth.
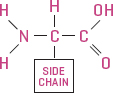
Plant proteins are made of combinations of twenty amino acids. Each amino acid has a central carbon (C) bound to a nitrogen-based amino group (NH2), a carboxyl group (COOH), a hydrogen atom (H), and a variable side chain group.
Proteins are large molecules, 10,000 to 100,000 times larger than a single hydrogen atom. They are composed of peptides, shorter chains of amino acids that are linked by peptide bonds, which allow proteins to bend, fold, and twist. Actually, it would more accurate to note that the order of the amino acids and how they are bonded (especially the first three) is what determines a protein’s shape. This folding is one reason so many can fit into a tiny cell and why they can move through cellular membranes and plasmodesmata. The folds and subsequent shapes of proteins can be affected by pH, temperature, and chemical signaling. Their shapes, along with the large size of protein molecules, make them well suited to serve as structural elements and to manipulate other molecules and atoms as enzymes. Carbohydrates may rule in the cell wall, but proteins are the basic building blocks of the cell itself. In fact, they play such an essential role that a tremendous amount of the DNA in a cell is dedicated to their synthesis.
All enzymes are proteins made by linking amino acids in a very specific order. Each plant cell produces thousands of different kinds of enzymes, each of which consists of hundreds to thousands of amino acid units linked together by peptide bonds. Once formed, enzymes take a unique shape as a result of their bonds and very long chains. These shapes allow them to carry out a specific reaction quickly. There are even enzymes to speed up the reactions that form new enzymes.
Enzymes can latch onto a specific molecule and either break it apart or make it bind to another. Try to imagine them folding and unfolding and twisting as they do their work. A single plant cell might have 10,000 different kinds of enzymes, and there may be 1 million copies of each in the cell. So, an enzyme is able to bump into the right molecules to fit together, which allows these reactions to happen so quickly. These protein enzymes are used to make lipids, carbohydrates, and nucleotides in the cell.
The names of enzymes usually end in -ase, as in lactase, the enzyme that breaks apart lactose. Proteases break down protein chains. Peptidases break peptide bonds to release amino acids. Lipids are broken down by lipases. (If there were a pill to reduce fat, it would surely be made of these.) Amylases break down starches, whose end products are sugars, and these are progressively broken down by maltase, lactase, and sucrase until the sugar monomer glucose is all that’s left. I could fill this book with the complex names of enzymes like phosphoglucomutase and pyrophosphorylase.
Enzymes are extremely crucial to all cellular activities, be they metabolic or synthetic. There is no life without them. It should not come as a surprise that once an important enzyme cannot be replicated, a cell will die. Remember, life in a cell (life in general, actually) is only a series of chemical reactions. If you don’t have enzymes to speed these up, you don’t have life.
Given their central role in the make-up of cellular membranes and the critical role these membranes play, it’s no wonder lipids are considered molecules of life. As with carbohydrates, proteins, and nucleic acids, lipids are characterized by special configurations of chains of atoms. The building blocks of these lipid chains are fatty acids, which are composed of carbon, hydrogen, and oxygen atoms. Lipids are classified into fats, oils, waxes, glycolipids (sugar added), phospholipids, lipoproteins, steroids, terpenes, and carotenoids.
Lipids are important and necessary molecules of life because they do not react to the special properties of water. Because there are no charges on these long chains (that is, they are nonpolar), lipid molecules will not dissolve when put into water. This is why oil floats to the top of water. In fact, lipids stick together (coagulate) to avoid water. This property allows them to work well in membranes in and around a cell.
In addition to phospholipid membranes, lipids play an important role in the storage of energy. Their long chains have lots of hydrogen bonds that release lots of energy when broken. (Fats have more energy in them than any other molecule of life.) This means they are a great food source to directly provide energy, as well as a great source for storing it.
Steroids, composed of four carbon rings attached to a long hydrocarbon chain, are lipids. These molecules are necessary to make hormones, which are signaling molecules produced by plant cells. Suberin is also a lipid. This is the waxy substance that clogs the cell walls in the Casparian strip at the end of the apoplastic pathway. In addition, the cutin on the outer layer of many plant epidermal cells is composed of lipids.
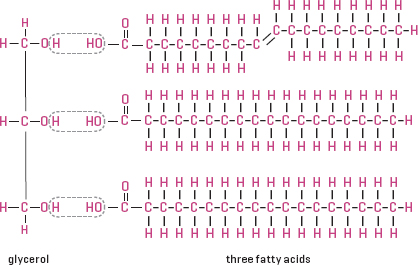
Lipids store energy and are used to make membranes. The basic structure of a lipid is long fatty acid chains bound to a glycerol or phosphate base.
Last, but not least, on the list of molecules of life are the nucleic acids, the two most famous of which are DNA and RNA. Carbohydrates, proteins, and lipids are useless unless they are put into some order and given some direction. The specific and replicable ordering of molecules is driven by DNA and RNA. Chemical reactions are what cells are about, and it is DNA and RNA that run those chemical reactions. One might argue where these originated, but there is not any argument that these two molecules enable life to replicate itself.
Nucleic acids also have building blocks, called nucleotides. Each nucleotide consists of a nitrogen base that has a phosphate and a sugar bonded to it. The proper names of DNA and RNA are deoxyribonucleic acid and ribonucleic acid. DNA has a double helix shape and RNA has a single helix, a result of the hydrogen bonds that connect the molecular strands and nucleotides in them. These two key nucleic acids differ only in that DNA has one less oxygen atom than RNA.
DNA is composed of four nucleotides: adenine, guanine, cytosine, and thymine. In RNA, thymine is replaced by uracil. Adenine always pairs with thymine (or uracil), and guanine always pairs with cytosine. Here is the key point of all life, at least on a secular, molecular level. DNA is nothing more than a double strand of molecules. Put everything else aside because it is as simple as that.
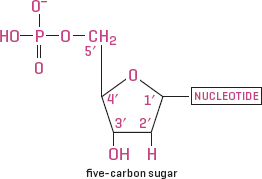
Combinations of nucleotides (nitrogen-based molecules) make up nucleic acids, whose general structure is one or more phosphate groups, a five-carbon sugar (deoxyribose or ribose), and a nucleotide.
DNA is composed of four nucleotides: adenine, thymine, guanine, and cytosine.
Nucleic acids are huge molecules. The DNA of the bacterium Escherichia coli, for example, has over 4 million pairs of nucleotides. All this DNA is tightly folded, of course; otherwise the molecule would be more than 1 millimeter (0.04 inch) long. Human DNA comprises 3 billion pairs of nucleotides. There’s a wide range in the numbers of nucleotides in the DNA of plants. Fritillaria assyriaca has 130 billion pairs, whereas Populus trichocarpa only has 480 million pairs. Even at the lower number, it’s amazing to think that each of these molecules is contained within a nucleus.
RNA is a single strand of nucleotides. It looks like half a ladder, one cut down the middle. That way it can match with another half or make one that is a reverse duplicate and fits. There are several different kinds of RNA. Messenger RNA (mRNA) transcribes the DNA pattern in the nucleus and then travels to one of the numerous ribosomes on the endoplasmic reticulum or floating free in the cytosol. Transfer RNA (tRNA) gathers the amino acids coded by the mRNA molecule from the cytosol. tRNA uses ATP to attach to the amino acids and take them to a ribosome. Tracks in the ribosome serve as guides to hold units in place so the newly placed amino acids remain in the proper sequence. Once finished, the constructed protein is released from the ribosome along with the mRNA, and the process starts all over again.
Each of the proteins in a cell has a unique sequence of amino acids. The constituent amino acids are lined up by RNA in ribosomes to be linked into polypeptide chains. Hundreds of RNA nucleotides and hundreds of enzymes are involved in assembling each protein in accordance with instructions copied from DNA. It is amazing to consider there are millions upon millions of proteins in every single cell of a plant, and each one is constructed on site.
Let’s tie in the molecules of life to the nutrients that plants take up. First, let’s consider the elemental composition of plant cell protoplasm, the stuff that’s in the nucleus and cytoplasm of a cell. The most abundant element is oxygen at 65 percent. The next highest is carbon at 18 percent, followed by hydrogen at 10 percent. Surprisingly, nitrogen accounts for only 3 percent of a plant cell, just a bit higher than calcium at 2 percent and phosphorus at 1 percent. Potassium, sulfur, chlorine, magnesium, and iron together account for only 0.9 percent, and zinc, boron, cobalt, molybdenum, copper, iodine, nickel, and manganese make up the remaining 0.1 percent.
These essential nutrients are used to build the molecules of life—not only those in the protoplasm but also in the complex protein- and carbohydrate-studded plasmalemma and its supportive cell wall. Proteins account for 7 to 10 percent of the weight of a cell. You might expect this, given how many enzymes there are in the cytoplasm and protein transporters in membranes. Carbohydrates weigh in at 2 or 3 percent and are mostly located in the liquid part of the cytoplasm (the hyaloplasm), cell wall, vacuole, and as storage molecules. Lipids, present in the membranes of cells and the hyaloplasm, account for 1 to 2 percent. Nucleotides, RNA, and DNA are located in the nucleus, mitochondria, chloroplasts, and cytoplasm and account for about 1 percent. The rest, between about 85 and 90 percent of a cell’s weight, is water. So, add water to these four kinds of molecules, and you have life!
 The Assimilation of Nitrogen I won’t take you on the journey to see how each of the essential nutrients becomes part of the molecules of life or are used to otherwise regulate their construction. However, because nitrogen is such an important nutrient, it makes sense to at least briefly follow its path once it passes through the plasmalemma.
The Assimilation of Nitrogen I won’t take you on the journey to see how each of the essential nutrients becomes part of the molecules of life or are used to otherwise regulate their construction. However, because nitrogen is such an important nutrient, it makes sense to at least briefly follow its path once it passes through the plasmalemma.
Again, nitrogen is assimilated into plants in two forms, as ammonium (NH4+) and nitrate (NO3−). When a plant takes in NH4+ and it enters into the cytoplasm of a cell, one of the H+ in its ionic structure quickly combines with an OH+ typically floating around due to the relatively high pH. The result is a water molecule and an ammonia molecule (NH3). This reaction changes the pH of the cytoplasm by decreasing the OH+ concentration (that is, increasing the H+), which can quickly create toxic conditions and mess with the transport of electrons needed for photosynthesis and respiration. In order not to have this happen, ammonia is either converted into organic molecules or transported to a vacuole, where the conditions are acidic, meaning there are plenty of H+ to return it to ammonium and thus a non-toxic state.
Nitrate is also converted into organic molecules. At first, this typically occurs in the roots, but as more and more nitrate accumulates, it is moved to shoot cells for assimilation. The conversion to organic molecules or assimilation causes NO3− to become nitrite (NO2−), which is toxic to plant cells. Nitrite molecules formed in roots are moved to plastids, and those formed in leaves are moved to chloroplasts. In each of these organelles, there is the enzyme nitrite reductase, which converts the nitrite to ammonium. This is then used to make glutamine, which is ultimately converted to glutamate. At this point nitrogen is available for use in different amino acids.
Rarely do gardeners stop to wonder at the fact that plants make all the molecules of life from just seventeen elements that are put together as a result of lots and lots of enzymatic activity, RNA, and DNA. It would take a college education to just scratch the surface of the various kinds of substances made in plants, not to mention understanding how they work (or how to pronounce their names).
Carbon, hydrogen, and oxygen atoms provide structure. Nitrogen, sulfur, and phosphorus atoms have special bonds capable of making uniquely shaped molecules, as well as storing and transferring energy. Potassium, calcium, and magnesium atoms are carriers and regulators of how the other nutrients get into the system. The other elements are used to make unique enzymes and other proteins or to assist in chemical reactions making various molecules.
In the nucleus are nucleic acids, which hold the instructions for how and when to put each and every single one of these atoms together into molecules. These instructions and the essential elements are used for absolutely everything from energy and structure to maintenance and growth. It is amazing that so few building blocks could result in such a wide diversity of the wondrous and complex organisms that plants are.
 Carbohydrate molecules consist of carbon, oxygen, and hydrogen and form large, repeating chains with lots of hydrogen bonds that store energy converted from sunlight.
Carbohydrate molecules consist of carbon, oxygen, and hydrogen and form large, repeating chains with lots of hydrogen bonds that store energy converted from sunlight.
 Proteins are large molecules made up of amino acids.
Proteins are large molecules made up of amino acids.
 There are about 100,000 different kinds of enzymes, special proteins that speed up cellular chemical reactions. Lack of key enzymes will cause cellular death.
There are about 100,000 different kinds of enzymes, special proteins that speed up cellular chemical reactions. Lack of key enzymes will cause cellular death.
 Lipids are composed of fatty acids. They are important in membranes because they do not react with water. Lipids also have many hydrogen bonds that make them ideal for storing energy.
Lipids are composed of fatty acids. They are important in membranes because they do not react with water. Lipids also have many hydrogen bonds that make them ideal for storing energy.
 Nucleic acids are made up of nucleotides and include DNA and RNAs.
Nucleic acids are made up of nucleotides and include DNA and RNAs.
 Around 85 to 90 percent of a cell is water. Proteins account for 7 to 10 percent; carbohydrates for 2 to 3 percent; lipids for 1 percent; and RNA, DNA, and the other nucleic acids for about 1 percent.
Around 85 to 90 percent of a cell is water. Proteins account for 7 to 10 percent; carbohydrates for 2 to 3 percent; lipids for 1 percent; and RNA, DNA, and the other nucleic acids for about 1 percent.
 Nitrogen enters the cell as ammonium or nitrate and is either converted to an organic molecule or moved to the vacuole. Nitrates are ultimately converted to glutamate.
Nitrogen enters the cell as ammonium or nitrate and is either converted to an organic molecule or moved to the vacuole. Nitrates are ultimately converted to glutamate.