13 Changing oceans
There is no doubt that one of the major environmental debates facing the human population is the potential effects of global climate change, sometimes referred to as global warming. Records from ice cores taken from the ice sheets in Greenland and the Antarctic show that climate change has occurred many times over hundreds of thousands of years, but it is the global climate changes since the 1850s that are foremost in the general public’s thinking, and that drive global environmental policy agendas. Sophisticated climate models are being employed to predict how much global temperatures will increase, and the current predictions range between 1.8 °C and 4 °C by about 2100. In this chapter three different (although often related) large-scale environmental changes will be introduced, and all have large impacts from the scale of an organism through to the entire globe (Figure 13.1).

Figure 13.1 The effect of large-scale environmental change impacts on many scales. On the organism scale it includes change in primary production, growth rate, reproduction, dispersal. On the population scale it includes change in population growth, species distribution and abundance. At the level of community/ecosystems change induces alteration to trophic interactions, biodiversity and community structure. Impacts at the scale of fisheries include changes in catches and fisheries management policies. On the global scale, human population growth and migration are impacted through both food and energy supply.
13.1 Global climate change
The consequences of global climate change for the oceans are many and varied, and tied up with complex interactions between air temperature, weather patterns, and ocean circulation. Here the effects of some of these on the Arctic Ocean will be used to illustrate the changes that are taking place in the oceans.
Since the early 1980s, average air temperatures at latitudes higher than 60 °N have increased by about 0.5 to 0.9 °C per decade. In general, springs are warmer and coming earlier in the year. A direct consequence of this warming trend is that some areas of the Greenland ice sheet have increased their rate of melting, and there has been increased melting of large glaciers in other Arctic regions. This melting has led, in part, to the 10 to 20 cm increase in sea level since 1900, or the current estimate of sea level rise of 3 mm per year.
NB Sea level is rising with increased input of fresh water from glaciers and ice sheets. This is not to do with melting pack ice (ice formed from frozen seawater) since sea ice is less dense than seawater, and when it floats on the ocean surface it only displaces its own weight of seawater (see Chapter 2). If all the sea ice in the earth melted, the melt water would simply replace the volume previously occupied by the ice. That is why there are no sea level changes with the annual formation and melting of the millions of square kilometres of sea ice each year.
It is not just increasing temperature itself that is causing the dramatic changes being recorded. Over the past few decades it has become clear that many oceanographic trends in the northern hemisphere, and the sea ice dynamics, are closely linked to the North Atlantic Oscillation (NAO) and the closely related Arctic Oscillation (AO). The NAO index is a measure of the atmospheric pressure differences between the region of Greenland/Iceland and the subtropical central north Atlantic in the Azores. The NAO index is defined as the difference between the Icelandic low and the Azores high in winter (December to March).
When the NAO index is positive there is a strong Icelandic low and Azores high pressure, with a corresponding strong North–South pressure gradient. During negative NAOs the pressure gradient is weak, with an Icelandic high and Azores low. The warming in the Arctic has been gradual over the past 100 years. However, the pace of this warming over the past 20 years has been increasing at a rate 8 times higher than the longer 100 year trend, and the rapid warming trends are thought to be associated with increasing positive phase in the NAO index (Figure 13.2).

Figure 13.2 Long-term variation in NAO index.
The most dramatic large-scale changes to be recorded in any marine system in the past 30 years are the changes in yearly minimum sea ice cover in the Arctic, which has been decreasing at a rate of about 6.8% per decade. The consequence is an annual decrease in mean annual sea ice cover of around 4% per decade. It is not only the extent that is reducing, but also the thickness of the ice: between the 1960s and 1990s there has been a decrease in sea ice thickness of more than 2 m in the central Arctic. These sorts of findings have led several researchers to conclude that there will be no summer sea ice in the Arctic after the 2050s. This would mean that although sea ice would form in winter, it would all melt in the following spring/summer, and there would be no thick pack ice, which was characteristic of the Arctic in the past. Clearly such dramatic changes in the seasonal dynamics of the physical structure of a whole ocean basin will have profound implications for the behaviour of the oceanography of the Arctic Ocean, and the seasonal dynamics of the ecosystem that has evolved around a sea ice cover lasting throughout the year.

Figure 13.3 Changes in sea ice extent in the Arctic. The ice conditions since 2007 have been consistently lower than that recorded by satellite measurements made in the period 1979–2000.

Figure 13.4 Detailed picture of changes in monthly ice extent between (A) July and (B) December between 1979 and 2011. The July trend shows a decrease of 6.8% per decade and the December trend a decrease of 3.5% per decade.

Figure 13.5 The sea ice and ice shelf in this image have high albedo (are highly reflective) whereas the seawater has a low albedo (absorbs energy)
Ice is a very good reflector of incidentsolar radiation, whereas seawater is a comparatively good absorber of heat energy from solar radiation. Ice and snow are said to have a high albedo, whereas the dark water has a low albedo. When ice melts, the albedo of the remaining ice is reduced, and therefore more energy can be absorbed. This in turn will increase the rate of melting. This is termed a positive albedo feedback loop where the absorption of heat energy leads eventually to an even greater absorption of energy. If there is an increased melting of sea ice in the Arctic, the reduced albedo will induce further warming of the surface waters, with thinner ice resulting in accelerated ice melt.
13.2 Ocean acidification
There are two contrasting issues for the carbon chemistry of the oceans in the future warming environment that is being predicted by climate scientists:
- As oceans warm, they will absorb less CO2 from the atmosphere, since the solubility of CO2 is less in warmer water.
- As more CO2 is absorbed by the oceans due to the rising concentrations in the atmosphere, there will be reduction in the pH of the seawater, a process commonly referred to as ocean acidification.
Since the Industrial Revolution in the 1700s it is estimated that the average pH of the oceans has dropped from 8.16 to 8.05. It is predicted that by 2100 the global oceans may have undergone a further decrease of about 0.4 pH units (Figure 13.6). Such changes are greater than any pH change thought to have happened at any time over the past 300 million years.
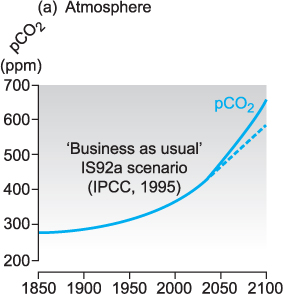

Figure 13.6 Predicted changes in atmospheric CO2 to the year 2100 and associated changes in ocean pH and carbon chemistry (cf. Chapter 9).
At the moment the surface of the oceans is saturated in the various mineral forms of calcium carbonate, and these do not dissolve at these depths, whereas they do dissolve in deeper waters that are under-saturated in these minerals. Because of the lowering of pH of ocean waters, and the associated reduction of carbonate ions, the surface waters are becoming less saturated in calcium carbonate. It is predicted that in the next 150 years the carbonate-saturated surface waters in some regions, especially cold Arctic and Antarctic waters, may disappear altogether. It is intriguing that in the Arctic, the organisms and ecosystems are not only being impacted by massive changes in sea ice dynamics, but also undergoing change due to pH.
There are many groups of organisms that use calcium carbonate in their body skeletons and/or external structures such as shells. These include the corals and molluscs, but also echinoderms, crustaceans, some seaweeds, foraminifers and calcareous phytoplankton such as the coccolithophores (Chapter 8). Naturally, anything that reduces the availability of carbonates for building these structures will impact on the organisms’ ability to grow and survive.
Currently there are many long-term experiments being conducted by marine biologists to see what the impacts will be, but there are now many reports of shells/skeletal structures being thinner/weaker when the organisms are kept in lower pH waters. Obviously if such effects occur in nature there will be significant impact on the populations of calcifying species. It is not simply a matter of taking adults and observing the changes that occur, but it is important to see the effects of lowered pH on all stages of life history, since some stages may be more vulnerable than others (e.g. larvae). This has been highlighted in experiments with fish, where the eggs were more vulnerable to high-CO2 induced mortality than the larval stages. Increased ocean acidification has also been shown to cause considerable tissue damage to larvae of the Atlantic cod (Gadus morhua), which clearly will impact on the stocks of these fish.
13.3 Eutrophication
As described in chapters 9 to 11, the growth of phytoplankton is limited by light, inorganic nutrients, and whether or not it is eaten by zooplankton. If there is a plentiful supply of the first two factors and no grazing, then the amount of algal biomass that grows in a water body can be very great indeed (Figure 13.7).

Figure 13.7 This shallow rock pool has been fertilised by the guano from birds and has lots of light, so the algal growth has been prolific. Compare the colour of the pool with that of the sea in the background.

Figure 13.8 River discharging into coastal waters that can transport the nutrients in run-off from agricultural land.
Over the past 100 years there has been a dramatic increase in the amount of nitrogen and phosphorus that has been applied to land to increase crop production, and this trend has sharpened especially in the past 20 years. Ultimately, much of this nitrogen and phosphorus runs off into rivers, and eventually through estuaries into coastal waters. The resulting increase in phytoplankton growth is well documented and is called eutrophication.
Although human-induced nutrient enrichment is the most common cause, eutrophication can result from a range of processes that increase the rate of supply of organic matter to an ecosystem, and is not confined to the increase of nutrient supply alone. So something that increases the light availability for photosynthesis, such as a reduction in the load of suspended mineral particles, could result in increased algal growth, and therefore cause eutrophication. A change in the residence time of water in an estuary could also lead to eutrophication through increased algal production. Eutrophication is generally perceived as a detrimental process, which can cause multiple harmful effects on an ecosystem stemming from increased production of organic matter within the system. Harmful effects can be divided into direct effects related to phytoplankton growth (e.g. increased biomass, algal blooms) and indirect effects (reduced biodiversity, increased biomass of opportunistic species, oxygen deficiency on the sea floor) caused by excess organic matter in the system. Eutrophication is typically a human-induced problem, and it is important to stress that it can also be reversed by proper management. There are also instances when low levels of eutrophication can even be considered as being a positive state for increasing the productivity of a water body or coastal system.

Figure 13.9 (A) Algal bloom in the Baltic Sea, which has been subjected to eutrophication for several decades.

Figure 13.9 (B) Surface water view of Baltic Sea cyanobacteria bloom in eutrophic coastal waters.
Where eutrophication does occur on a large scale, and over sustained periods of time, the consequences for the ecosystem can be dramatic. This is especially true of enclosed, or semi-enclosed and permanently stratified water bodies such as the Baltic Sea. The increases in phytoplankton growth in the Baltic have been associated with increased turbidity of the water reducing the light available for growth of submerged seaweeds and plants. Also the diversity of phytoplankton species has changed, with different species now dominating the communities than in the past. However, the most dramatic change has resulted from the sinking of the increased phytoplankton blooms to the sea floor. The Baltic Sea has a permanent salinity stratification, which slows the supply of oxygen to near-bottom waters. The increased breakdown of organic matter has led to such an increase in respiration that the oxygen concentrations close to the seabed have become very low in places, and in some regions so low that benthic organisms have been killed.