
We know that two charges exert either an attractive or repulsive force on each other, but what is the nature of this force? It turns out that the force between any two charges follows the same basic form as Newton’s law of universal gravitation; that is, the electric force is proportional to the magnitude of the charges and inversely proportional to the square of the distance between the charges. The equation for Coulomb’s law is

where FE is the electric force, q1 and q2 are the charges, r is the distance between their centers, and K is a constant that happens to equal 9 × 109 Nm2/C2. Usually, you will be asked questions about the proportionality between the quantities in Coulomb’s law, rather than the equation.
Example: Two point charges q1 and q2 are separated by a distance r.
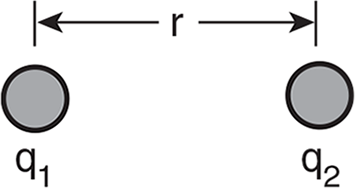
The following choices refer to the electric force on these two charges.
Solution:
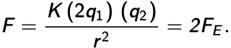
Thus, the new force between the charges is doubled, answer (b).
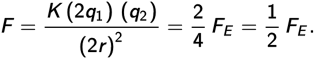
Thus, the new force is half as much as the original force, answer (d).