Answers and Explanations
Review Questions
- B
The average kinetic energy of the molecules is proportional to the temperature of the substance. - DKelvin = Celsius + 273, so 0 K = –273°C.
- C
Joule first measured the mechanical equivalent of heat. - A
The expansion of a substance is proportional to the change in temperature. - B
Convection is heat transfer by the rising and falling of a fluid as it heats and cools and changes density. - C
The fire radiates heat mostly in the form of infrared electromagnetic waves. - B
Three times the mass of the same substance will require three times the heat to raise its temperature by a certain amount according to the equation Q = mcΔT. - D
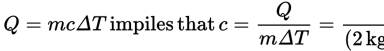
- C
The heat added to the ice is used only to change the phase of the ice to water, and the heat added to the liquid water is used to change its temperature. - A
The first law of thermodynamics states that the heat lost by one object must be gained by another, but the amount of energy in the transfer must remain constant. - D
If only 15 J of the 60 J is used to do work, we say that the efficiency of the process is
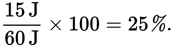
- E
Entropy means disorder, and every natural system will tend toward a state of higher disorder. The fact that heat spontaneously flows from a hotter body to a colder body is a consequence of this law.