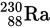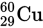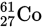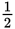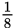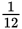Review Questions
-
The neutral element magnesium
 has
has- 12 protons, 12 electrons, and 24 neutrons.
- 12 protons, 12 electrons, and 12 neutrons.
- 24 protons, 24 electrons, and 12 neutrons.
- 24 protons, 12 electrons, and 12 neutrons.
- 12 protons, 24 electrons, and 24 neutrons.
-
All isotopes of uranium have
- the same atomic number and the same mass number.
- different atomic numbers but the same mass number.
- different atomic numbers and different mass numbers.
- the same atomic number but different mass numbers.
- no electrons.
-
Six protons and six neutrons are brought together to form a carbon nucleus, but the mass of the carbon nucleus is less than the sum of the masses of the individual particles that make up the nucleus. This missing mass, called the mass defect, has been
- converted into the binding energy of the nucleus.
- given off in a radioactive decay process.
- converted into electrons.
- converted into energy to hold the electrons in orbit.
- emitted as light.
-
The isotope of thorium
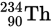 undergoes alpha decay according to the equation
undergoes alpha decay according to the equation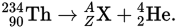
The element X is
-
-
The isotope of cobalt
 undergoes beta decay according to the equation
undergoes beta decay according to the equation
The element X is
-
-
The half-life of a certain element is 4 years. What fraction of a sample of that isotope will remain after 12 years?
-
-
Consider the following nuclear equation:

This equation describes the process of
- radioactivity.
- fission.
- fusion.
- electron energy level transitions.
- reduction and oxidation.