Introduction
The chemical diversity of wine
How many choices does a consumer have when they buy a wine? In the United States, all wines sold must have a Certificate of Label Approval (COLA) from the Alcohol and Tobacco Tax and Trade Bureau (TTB), and in 2013 the TTB approved over 93 000 COLA requests.1 Because many wines are vintage products, that is, a new label will be produced for each harvest year, the true number of wines available in wine stores throughout the United States may be closer to 250 000.2 In contrast to commodity products where producers strive for homogeneity (e.g., soybeans, milk), variation in specialty products like wine is not only tolerated – it is appreciated and celebrated. Consumers expect that wines with different labels should smell different, taste different, and look different; from a chemist’s perspective, consumers expect wines to have different chemical compositions. The study of wine chemistry is the study of these differences – explaining how there can be hundreds of thousands, if not millions, of different wine compositions, and contributing to a winemaker’s understanding of how the myriad of choices they are faced with can lead to these differences.
What is wine?
To a first‐order approximation, a dry table wine is a mildly acidic (pH 3–4) hydroalcoholic solution. The two major wine components are water and ethanol, typically accounting for about 97% on a weight‐for‐weight (w/w) basis. The remaining compounds – responsible for most of the flavor and color of wine – are typically present at < 10 g/L (Figure I.1), and many key odorants are found at part‐per‐trillion (ng/L) concentrations! Notably, none of these compounds appear to be unique to wine – compounds present in wine can also be found in coffee, beer, bread, spices, vegetables, cheese, and other foodstuffs.3 What distinguishes different wines from other products (and each other) is differences in the relative concentrations of compounds, rather than the presence of unique components.
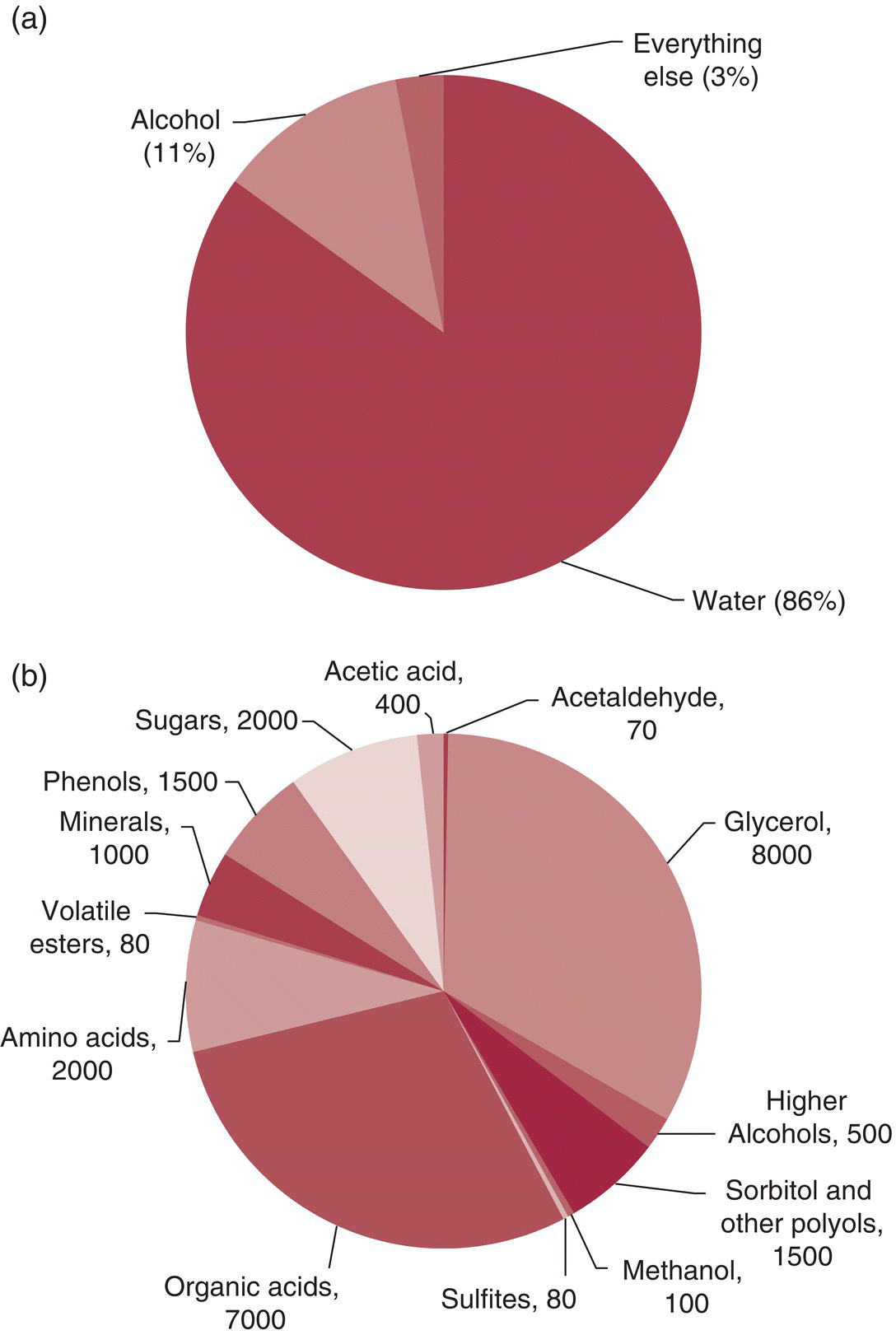
Figure I.1 Composition of a representative dry red table wine (a) on a % w/w basis and (b) typical concentrations (mg/L) of major wine components excluding water and ethanol, that is, the main contributors to “Everything Else.” Key trace components (0.1 ng/L–10 mg/L) would not be visible and are therefore not included
Wine is produced by the alcoholic fermentation of grape juice or must (juice and solids), which results in the complete or partial transformation of grape sugars to ethanol and CO2. However, winemaking and wine storage result in many chemical changes beyond simply the consumption of sugars and formation of alcohol. This is readily exemplified by the volatile composition of a wine, which is far more complex than that of grape juice (Figure I.2). These volatile components can contribute to the aroma of wine and such odorants are often classified based on when they are formed; that is, in the grape (primary), during fermentation (secondary), or during storage (tertiary) (Table I.1).
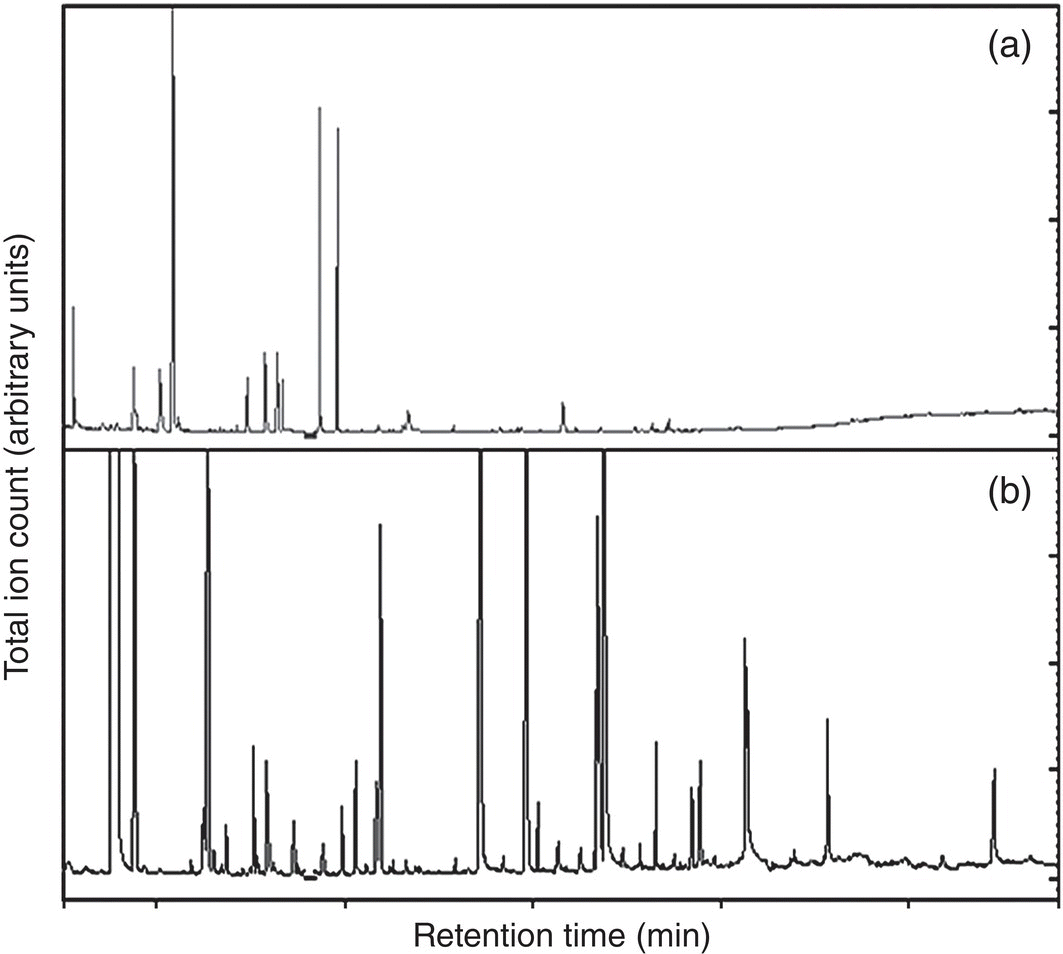
Figure I.2 Comparison of GC‐MS chromatograms for (a) a grape juice and (b) a wine produced from that grape juice. Every peak in the chromatograms represents at least one unique volatile compound
Table I.1 Primary, secondary, and tertiary classifications of wine odorants
| Compound classification | Description | Examples (Part and Chapter) |
| Primary | Compounds present in the grape that persist unchanged into wine | Methoxypyrazines (A.5), rotundone (A.8) |
| Secondary | Compounds formed as a result of alcoholic or malolactic fermentation due to either
|
|
| Tertiary | Compounds formed during wine storage, for example, as a result of
|
|
The number of compounds identified in wine follows advances in analytical technology. A survey from 1969 reported that wine and other alcoholic beverages contained 400 volatiles, while a later book from 1983 reported over 1300 volatiles [1]. A more recent analysis of wines using a state‐of‐the‐art mass spectrometry system (FT‐ICR‐MS) was able to detect tens of thousands of unique chemical signals across a set of wines, and assign chemical formulae to almost 9000 components [2]. However, the advanced instrumentation in this last report would not distinguish structural isomers – for which there may be billions for a condensed tannin consisting of 30 monomers (Chapter 15). Thus, the number of chemical compounds in wine, like most natural products, is essentially uncountable.
With this in mind, the goal of a wine chemist is not to enumerate every compound, but rather to identify compounds, or in many cases classes of compounds, that will directly or indirectly control key quality aspects of the wine such as organoleptic properties (aroma, flavor, appearance), safety, and stability. Alternatively, compounds may be of interest because they can be used to detect the presence of fraud. These categories, and examples, are summarized in Table I.2.
Table I.2 Summary of major functional classes of interest to wine chemists. Note that compounds may fit into more than one category
| Compound functions | Description | Examples (Part and Chapter) |
| Organoleptic | Compounds that contribute to the taste, odor, or tactile sensations of a wine Compounds that affect wine color or cause a visible haze Compounds that act as precursors of organoleptically active compounds |
Acids (A.3), monoterpenes (A.8), tannins (A.14) Anthocyanins (A.16), proteins (B.26.2) Glycosides (B.23.1), S‐conjugates (B.23.2) |
| Stability | Compounds that inhibit or promote microbial or abiotic changes during storage | Organic acids (A.3), sulfur dioxide (A.17) |
| Bioactive | Compounds that may positively or negatively affect human health | Phenolic compounds (A.11), biogenic amines (A.5), ethyl carbamate (A.5) |
| Matrix | Compounds that affect the speciation or activity of other compounds, usually through non‐covalent interactions | Water and ethanol (A.1) |
| Authenticity | Markers that help distinguish authentic products from fraudulent products | Artificial colors (C.28) |
Chemical reactions in wine
The complexity of its composition would suggest that the range of chemical reactions in wine is limitless. However, as noted above, wine is ~97% ethanol and water, which precludes the large number of reactions in introductory organic chemistry texts that require the absence of protic solvents (e.g., no Grignard reactions). Similarly, the mildly acidic conditions of wines (typically, pH ~3.5) mean that base‐catalyzed reactions are usually of low importance (e.g., aldol condensations are unlikely).
As with all chemistry, the key to predicting reactions is to define the components of wine that can react with each other. Many of these reactions will be familiar to students of organic chemistry, and include:
- Reactions between nucleophiles and electrophiles, for example, bisulfite and carbonyls
- Hydrolytic reactions, usually acid‐catalyzed, for example, of esters, interflavan bonds, and glycosides
- Addition and elimination reactions, again usually acid‐catalyzed.
These reactions and many more are the very essence of this book, and are presented in detail throughout the following chapters. One uniquely challenging aspect of wine chemistry as compared to the organic chemistry lab (and most other foodstuffs) is that reactions are allowed to take place for months, years, or even decades, often at ambient temperatures and in a reductive environment. These conditions can lead to unexpected reaction products – this is especially important since a part‐per‐trillion of certain compounds may be enough to affect flavor.
Chemistry as a historical record
Many chapters of this text, particularly in Part A, contain tables of “typical concentrations” of various wine components, usually from peer‐reviewed reports published since 2000.4 However, grapegrowing and winemaking practices are not static [3], and typical values may change dramatically with changes in fashion or technology – not to mention climate [4]. In some cases, the analysis of aged wine reveals changes in typical wine composition and lends insight to changes in production practices. For instance, in the nineteenth century, wine drinkers used to prefer much sweeter wines – premium Champagnes would have over 140 g/L of sugars, as compared to < 10 g/L in most modern versions [5]. Control of spoilage organisms like acetic acid bacteria through the use of sulfur dioxide and anaerobic storage during this period was still primitive – a survey of Greek wines from 1872 reported acetic acid concentrations in the range of 1.5 to 3.6 g/L [6], all in excess of modern legal limits. Wine tanks from this era also typically had lead‐containing bronze valves (no longer used today), which could be leached under acidic conditions to result in relatively high lead concentrations in wine [5]. Differences in mineral content could also arise from viticulture practices – grapegrowers in the nineteenth and early twentieth century routinely used arsenic to ward off insects and mold [7].5
In summary, the values provided in this text should be seen as a snapshot of wine composition circa the early twenty‐first century, rather than as fundamental constants. A modern consumer’s expectation of a high‐quality wine is a reflection of both improved technical capacities as well as accumulated traditions, which in the year 2016 means (for the majority of internationally known premium wines) fermenting with selected strains of Saccharomyces cerevisiae, aging in oak barrels, and storing wine in glass bottles with corks. Presumably, the pursuit of different options in the past might have led to alternative perspectives on the idea of wine “perfection” and target chemical compositions. Similar statements can be made about the future of wine, and we expect that the numbers provided here will provide some amusement to the wine chemists of the year 2100.
The chemical senses and wine flavor
The majority of compounds discussed in this text have a role in wine flavor. Because the lexicon used to discuss flavor in scientific publication differs from that used in casual conversation, this introduction will conclude with a brief review of key flavor terminology.
Flavor is defined as the “perception resulting from stimulating a combination of the taste buds, the olfactory organs, and chemesthetic receptors within the oral cavity” [10] – in other words, everything a taster can perceive in the mouth, for example, olfaction, taste, and chemesthesis.
Olfaction, or smell, involves the detection of odorants by olfactory receptors (ORs) located within the nasal cavity. Humans have approximately 700 OR, of which half are usually functioning in any individual [11]. Although each specific OR demonstrates some selectivity towards compound classes, odorants (or mixtures of odorants) typically stimulate combinations of OR, and these combinatorial patterns are associated with particular smells [12]. Olfaction requires that the odorant be volatile to reach the nasal cavity, a process that may occur through two routes:
- Orthonasal olfaction involves the detection of odorants without tasting, for example, by smelling the headspace of the wine. The perceptions arising from orthonasal olfaction are often referred to as aroma.
- Retronasal olfaction involves detection of odorants that travel from the oral cavity to the nasal cavity. Most commonly, this occurs following swallowing, after which exhalation drives a small amount of odorants through the nostrils [13].
Although olfaction is selective for volatile compounds, most food volatiles appear to be unimportant to odor. A recent meta‐analysis estimated that of the ~10 000 volatiles detected in foodstuffs, <3% were important to food aroma [12]. The same review noted that the aromas of specific foods or beverages (including wine) could be simulated with between 4 and 44 odorants.
Taste involves the detection of small molecules by taste receptors located in the taste buds. Five classes of taste receptors have been established – “sweet,” “sour,” “bitter,” “salty,” and “umami” [14], of which only the first three appear to be routinely experienced in wine [15].
Chemesthesis involves the chemical activation of receptors responsible for sensations of pain, temperature, and touch, for example, the “heat” caused by capsaicin in hot chili peppers [16]. There are several critical distinctions between taste and chemesthesis, the most important being that taste is only sensed by the taste receptors of the tongue, while chemesthesis can be detected throughout the oral cavity – and for that matter, throughout the body.6 The chemesthetic sensations most important to wine are those that cause:
- Pungency and irritation, which can be due to ethanol and CO2.
- Astringency, or the perceived loss of lubrication in the mouth, which can be triggered by condensed tannins and other phenolic compounds [17].
The perception of “body” is also likely a result of chemesthesis, although the specific compounds responsible for this sensation are still unclear [18].
Classic papers on food analysis (grapes, wine, and otherwise) often focused on identifying or measuring the compounds found in high concentrations, with little emphasis on the sensory relevance of the compounds [19].7 Since the 1990s, it has been increasingly common to identify organoleptically important compounds through the use of bioassays, for example, using a human sniffer to identify key odorants through GC‐olfactometry. Candidate compounds can then be quantified and their relevance evaluated through reconstitution and omission experiments [20].
Perception of flavors. Although the eventual goal of bioassay‐based approaches is to recreate the organoleptic property in a model system, a key feature is the use of activity values as a rough estimate of a compound’s importance. Activity values are calculated as the ratio of a compound’s concentration to its sensory threshold in an appropriate matrix:
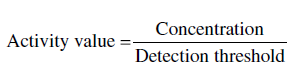
Typically, compounds with higher activity values have more intense flavors, but the concentration–response function varies among compounds. In simple solutions, the intensity of most taste compounds (sugars, acids) scales as a linear function of their concentration, but the intensity of most odorants increases as roughly the square root of concentration [16].
Use of activity values in evaluating the relevance of odorants to a given foodstuff dates to at least the 1960s [21, 22], and even earlier examples exist for other flavor compounds.8 As a general rule, compounds with activity values <1 are expected to have a negligible effect on a particular sensory attribute [20]. The utility of the activity value concept can be appreciated from representative data for Sauvignon Blanc wines in Table I.3. Based strictly on concentrations, 1‐hexanol appears to be a very important contributor to wine. However, conversion to odor activity values (OAVs) reveals that the “grapefruit” aroma of 3‐mercaptohexanol and the “herbaceous” notes of 3‐isobutyl‐2‐methoxypyrazine are far more likely to contribute to Sauvignon Blanc aroma – and, in fact, they do.
Table I.3 Concentrations, odor thresholds, and calculated OAVs for five representative compounds in Sauvignon Blanc wines
| Compound | Typical aromas | Concentration range (mg/L)a | Odor threshold (mg/L)b |
Odor activity value, OAV |
| Water | – | ~850 000 | – | 0 |
| 3‐Methylbutanol (Isoamyl alcohol) | Solvent, burnt | 200–250 | 30 | 6–8 |
| 1‐Hexanol | Grassy | 1.5–2.5 | 8 | 0.2–0.3 |
| 3‐Mercaptohexanol | Passionfruit, grapefruit | 0.0005–0.0038 | 0.000060 | 9–65 |
| 3‐Isobutyl‐2‐methoxypyrazine | Bell pepper | 0.000008–0.000023 | 0.000002 | 4–11 |
a Range of average values observed across 7 wine regions for 2004 and 2005 vintages [23].
b From References [24] to [26].
Activity values are useful as an initial screen to determine the likely relevance of a given compound. However, simply knowing whether a flavor compound is or is not present at suprathreshold concentrations (activity value > 1) is insufficient to determine if the compound is important to the foodstuff for several reasons:
- Masking. The perceived intensity of a flavorant can be decreased by the presence of other flavor compounds. For example, addition of herbaceous smelling methoxypyrazines to red wine decrease the intensity of fruity aromas [27].
- Additive or synergistic effects. Groups of homologous compounds, that is, series of alkyl esters or ketones, can reach sensory threshold through additive effects even if all compounds individually have activity values < 1 [28]. Synergism, that is, an increase in stimulus intensity beyond what is predicted for simple additive effects, can also occur, most often for taste and tactile sensations [16].
- Matrix effects. Differences in the matrix (pH, temperature, ethanol concentration, non‐covalent interactions with macromolecules) can change the activity of flavor compounds, and particularly the volatility of odorants [29].
- Synesthesia and confirmation bias. The different chemosensory modalities (taste, smell, tactile) do not operate in isolation; information from these senses is integrated together. For example, panelists report that increasing the sweetness of a fruity beverage increases the intensity of fruit flavor [30]. A related concept is confirmation bias, in which prior knowledge of a product affects a panelist’s perceptions; for example, white wines dyed with tasteless red food coloring are perceived as having fuller body [31].
- Emergent properties. Combinations of flavor compounds (particularly odorants) often elicit different percepts than individual compounds. For example, no specific wine compound has a smell exactly like wine, but the combination of odorants at appropriate concentrations allows a sniffer to know that they are smelling wine and not another beverage [32].
Finally, the use of a single value for a sensory threshold obscures the fact that individuals show considerable variation in their sensitivities to different flavor compounds, particularly odorants. One author estimated that a typical 96% confidence interval for odorant thresholds across a population spans a concentration factor of 256 [33], and individuals’ thresholds and descriptors may change with repeated experiences [34]. Although this variation does not preclude studies of organoleptic properties, it does necessitate appropriate sensory practices and rigorous statistical analysis of the data (as with other studies involving human subjects). A full discussion of sensory techniques is beyond the scope of this text, but its omission should not be interpreted as trivialization of sensory science. Collecting and interpreting sensory data can be laborious and often represents a limiting step in wine chemistry. We strongly encourage the reader to consult one of the many excellent texts available on sensory science to learn more (e.g., Reference [16]).
References
- 1. Nykänen, L. and Suomalainen, H. (1983) Aroma of beer, wine, and distilled alcoholic beverages, D. Reidel, Dordrecht, Holland.
- 2. Roullier‐Gall, C., Witting, M., Gougeon, R.D., Schmitt‐Kopplin, P. (2014) High precision mass measurements for wine metabolomics. Frontiers in Chemistry, 2, 102.
- 3. McGovern, P.E. (2003) Ancient wine: the search for the origins of viniculture. Princeton University Press, Princeton, NJ.
- 4. Mira de Orduña, R. (2010) Climate change associated effects on grape and wine quality and production. Food Research International, 43 (7), 1844–1855.
- 5. Jeandet, P., Heinzmann, S.S., Roullier‐Gall, C., et al. (2015) Chemical messages in 170‐year‐old champagne bottles from the Baltic Sea: revealing tastes from the past. Proceedings of the National Academy of Sciences of the United States of America, 112 (19), 5893–5898.
- 6. Thudichum, J.L.W. and Dupré, A. (1872) A treatise on the origin, nature, and varieties of wine; being a complete manual of viticulture and oenology, Macmillan, London.
- 7. Parascandola, J. (2012) King of poisons: a history of arsenic, Potomac Books, Herndon, VA.
- 8. Michel, R.H., McGovern, P.E., Badler, V.R. (1993) The first wine & beer. Analytical Chemistry, 65 (8), 408A–413A.
- 9. Guasch‐Jane, M.R., Andres‐Lacueva, C., Jauregui, O., Lamuela‐Raventos, R.M. (2006) The origin of the ancient Egyptian drink Shedeh revealed using LC/MS/MS. Journal of Archaeological Science, 33 (1), 98–101.
- 10. Anonymous (2009) E253‐09a, Standard terminology relating to sensory evaluations of materials and products, ASTM International, West Conshohocken, PA.
- 11. DeMaria, S. and Ngai, J. (2010) The cell biology of smell. The Journal of Cell Biology, 191 (3), 443–452.
- 12. Dunkel, A., Steinhaus, M., Kotthoff, M., et al. (2014) Nature’s chemical signatures in human olfaction: a foodborne perspective for future biotechnology. Angewandte Chemie International Edition, 53 (28), 7124–7143.
- 13. Buettner, A., Beer, A., Hannig, C., Settles, M. (2001) Observation of the swallowing process by application of videofluoroscopy and real‐time magnetic resonance imaging – consequences for retronasal aroma stimulation. Chemical Senses, 26 (9), 1211–1219.
- 14. Chandrashekar, J., Hoon, M.A., Ryba, N.J.P., Zuker, C.S. (2006) The receptors and cells for mammalian taste. Nature, 444 (7117), 288–294.
- 15. Hufnagel, J.C. and Hofmann, T. (2008) Orosensory‐directed identification of astringent mouthfeel and bitter‐tasting compounds in red wine. Journal of Agricultural and Food Chemistry, 56 (4), 1376–1386.
- 16. Lawless, H.T. and Heymann, H. (2010) Sensory evaluation of food principles and practices. Springer, New York.
- 17. Schöbel, N., Radtke, D., Kyereme, J., et al. (2014) Astringency is a trigeminal sensation that involves the activation of G protein–coupled signaling by phenolic compounds. Chemical Senses, 39 (6), 471–487
- 18. Runnebaum, R.C., Boulton, R.B., Powell, R.L., Heymann, H. (2011) Key constituents affecting wine body – an exploratory study. Journal of Sensory Studies, 26 (1), 62–70.
- 19. Schreier, P., Drawert, F., Junker, A. (1976) Identification of volatile constituents from grapes. Journal of Agricultural and Food Chemistry, 24 (2), 331–336.
- 20. Grosch, W. (2001) Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chemical Senses, 26 (5), 533–545.
- 21. Guadagni, D.G., Buttery, R.G., Harris, J. (1966) Odour intensities of hop oil components. Journal of the Science of Food Agriculture, 17 (3), 142–144.
- 22. Rothe, M. and Thomas, B. (1963) Aromastoffe des brotes. Zeitschrift für Lebensmittel‐Untersuchung und Forschung, 119 (4), 302–310.
- 23. Benkwitz, F., Tominaga, T., Kilmartin, P.A., et al. (2012) Identifying the chemical composition related to the distinct aroma characteristics of New Zealand Sauvignon Blanc wines. American Journal of Enology and Viticulture, 63 (1), 62–72.
- 24. Guth, H. (1997) Quantitation and sensory studies of character impact odorants of different white wine varieties. Journal of Agricultural and Food Chemistry, 45 (8), 3027–3032.
- 25. Tominaga, T., Baltenweck‐Guyot, R., Des Gachons, C.P., Dubourdieu, D. (2000) Contribution of volatile thiols to the aromas of white wines made from several Vitis vinifera grape varieties. American Journal of Enology and Viticulture, 51 (2), 178–181.
- 26. Buttery, R.G., Seifert, R.M., Guadagni, D.G., Ling, L.C. (1969) Characterization of some volatile constituents of bell peppers. Journal of Agricultural and Food Chemistry, 17 (6), 1322–1327.
- 27. Hein, K., Ebeler, S.E., Heymann, H. (2009) Perception of fruity and vegetative aromas in red wine. Journal of Sensory Studies, 24 (3), 441–455.
- 28. Guadagni, D.G., Buttery, R.G., Okano, S., Burr, H.K. (1963) Additive effect of sub‐threshold concentrations of some organic compounds associated with food aromas. Nature, 200 (4913), 1288–1289.
- 29. Pozo‐Bayón, M.Á. and Reineccius, G. (2009) Interactions between wine matrix macro‐components and aroma compounds, in Wine chemistry and biochemistry (eds Moreno‐Arribas, M.V. and Polo, M.C.), Springer, New York, pp. 417–436.
- 30. King, B.M., Duineveld, C.A.A., Arents, P., et al. (2007) Retronasal odor dependence on tastants in profiling studies of beverages. Food Quality and Preference, 18 (2), 286–295.
- 31. Delwiche, J. (2004) The impact of perceptual interactions on perceived flavor. Food Quality and Preference, 15 (2), 137–146.
- 32. Ferreira, V., Ortin, N., Escudero, A., et al. (2002) Chemical characterization of the aroma of Grenache rosé wines: aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. Journal of Agricultural and Food Chemistry, 50 (14), 4048–4054.
- 33. Amoore, J.E. (1980) Properties of the olfactory system, in Odorization (eds Suchomel, F.H. and Weatherly, J.W.), Institute of Gas Technology, Chicago, IL, pp. 31–35.
- 34. Stevens, J.C., Cain, W.S., Burke, R.J. (1988) Variability of olfactory thresholds. Chemical Senses, 13 (4), 643–653.