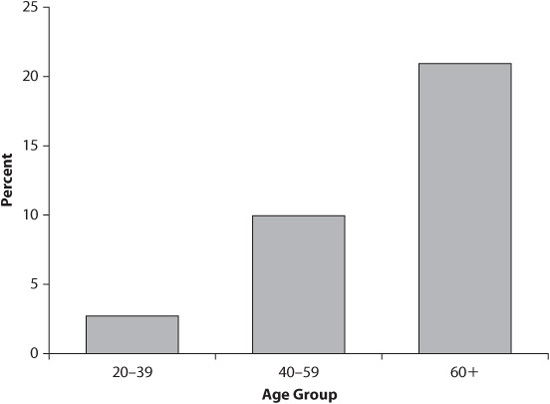
Numerous health problems can lead to kidney failure. The four main causes are diabetes, hypertension, glomerular diseases, and polycystic kidney disease (PKD). Genetics plays a role in most causes of kidney failure, and kidney failure may result from a specific defect inherited from one or both parents. PKD is primarily an inherited kidney disease.
Genetics is not the whole story, however. Lifestyle and other environmental factors may also be significant influences. This chapter begins with a primer on the genetic and environmental factors contributing to kidney failure. Knowledge of these factors will help you understand how you might slow the progression of your disease.
All of the functions in our bodies operate on the basis of instructions embedded in our genetic code. Over the past fifty years, researchers have uncovered the intricate details of how this genetic code works. The Human Genome Project, which was completed in 2003, determined the complete nucleotide sequence of human deoxyribonucleic acid (DNA).
Genes provide a blueprint for creating our bodies and making them work. Just as the blueprints for a house show how to build its various parts, like the foundation, walls, and roof, genes direct the construction of cells, organs, bone, and skin. Moreover, like the heating, air conditioning, and electrical systems that control the environmental conditions in a house, genes control how our bodies function.
The primary purpose of genes is making proteins. Proteins are like a building’s construction workers and engineers. They make and operate the human body according to the genetic blueprints residing within the chromosomes.
For all organs of the body, our genes issue the instructions (or blueprints) to make proteins while we are in the womb. For organs to develop correctly, certain processes must happen in an exact way. It starts when a sperm fertilizes the egg. The cells in the resulting embryo, possessing two copies of each gene, one from each parent, begin to divide. As the embryo grows, copies of genes inherited from each parent must be reproduced identically in each new cell, so that all the cells in the developing fetus (and eventually in the person’s body) will have the same set of genetic blueprints. During a person’s lifetime, many of the cells in the body will die and be replaced with new ones. These new cells generally also contain the same blueprints.
Although rare, mistakes can occur when genes are copied. Called mutations, these mistakes can cause the organs to work improperly. If a house’s blueprints are wrong, a door might be located in the wrong place or the lighting system might fail because of incorrect wiring. In people, gene mistakes are passed along to their children and can cause them to inherit a disease. For example, mutations in genes that make or control kidneys can malfunction, leading to disease.
Behavioral and environmental factors can also contribute to the expression or progression of a disease. In the case of kidney failure, an improper diet and lack of exercise or other lifestyle factors can contribute greatly to a person’s medical status. We do not deliberately set out to make ourselves ill. However, with the stresses of our culture and everyday life, it can be easy to neglect our own health. Between work, family, and social obligations, we are so busy that we may have little time to eat properly or to get adequate exercise. Over time, our health can begin to fail without our even knowing it.
When people don’t eat right and don’t exercise, they are more likely to be overweight or obese. Obesity has reached epidemic proportions in the United States and in other developed countries. According to a recent study, 66.3 percent of Americans are overweight, obese, or morbidly obese.1 African Americans and Hispanic Americans have a higher prevalence of obesity than non-Hispanic whites. Moreover, women across all races are more obese than men. Obesity increases with age, leveling off by age 60 or declining thereafter.
Obesity may lead to other health complications—including kidney failure—because obesity makes people more likely to develop diabetes and hypertension. Excess weight can also cause coronary heart disease, high cholesterol levels, and stroke, which can lead to death.
References to the symptoms of kidney disorders by the ancient Greeks suggest that we have known about kidney failure for thousands of years. We weren’t able to analyze kidneys and other organs until the nineteenth century, however. In 1827, the English physician Richard Bright first described the symptoms of kidney failure.
In the twenty-first century, kidney failure is still incurable, but it can be prevented and treated. With dialysis and transplantation, people with kidney failure can continue to have productive lives. Nevertheless, failing kidneys take a very high medical, emotional, and financial toll. There is no cure for kidney failure, but knowing its causes can help prevent, delay, or prepare for it.
As we learned in chapter 1, approximately one-half million people in the United States are living with kidney failure. In most cases, diabetes and hypertension are the causes. Both are preventable (see chapter 5). Glomerular disorders, which can have both environmental and genetic origins, are another cause of kidney failure. There are also inherited causes of kidney failure, like PKD. If a person inherits mutated genes, the disease will develop, although the progression of the disease varies among families and individuals. This chapter presents a brief overview of each of these four leading causes of kidney failure.
Diabetes (also known as diabetes mellitus) is the leading cause of kidney failure in the United States and accounts for 38 percent of cases. Because of rising obesity rates, diabetes rates are increasing, even among children. As many as 20.6 million people have diabetes. As people age, they become more susceptible to diabetes (see figure 3.1). About one-half of all people with diabetes are over 60 years old. Across the population, slightly more men than women have diabetes, and it disproportionately affects Native Americans, non-Hispanic African Americans, and Hispanic Americans (see figure 3.2). Understanding the underlying causes of diabetes is essential for learning how to prevent and treat it.
Figure 3.1. Estimated Total Prevalence of Diabetes in People Aged 20 Years and Older, by Age Group, United States, 2005
Source: 1999–2002 National Health and Nutrition Examination Survey estimates of total prevalence (both diagnosed and undiagnosed were projected to year 2005).
Diabetes is a metabolic disease in which the body does not properly utilize glucose. Glucose is the main source of energy in the body. In order for glucose to enter cells and produce energy, the pancreas secretes the protein insulin into the bloodstream to help glucose cross the membranes surrounding cells. If this process is interrupted, glucose accumulates in the blood and can spill out into the urine. Cells can starve without glucose, even with high concentrations of glucose in the blood, if it cannot permeate cell membranes.
Figure 3.2. Estimated Age-Adjusted Total Prevalence of Diabetes in People Aged 20 Years and Older, by Race/Ethnicity, United States, 2005
Source: For American Indians/Alaskan Natives, the estimate of total prevalence was calculated using the estimate of diagnosed diabetes from the 2003 outpatient database of the Indian Health Service and the estimate of undiagnosed diabetes from the 1999–2002 National Health and Nutrition Examination Survey. For the other groups, 1999–2002 NHANES estimates of total prevalence (both diagnosed and undiagnosed) were projected to year 2005.
The inability of insulin to process glucose efficiently can occur for one of two reasons: (1) a lack of sufficient insulin secretion by the pancreas or (2) a body’s resistance to insulin, preventing the transport of glucose into the cells. When the pancreas does not secrete enough insulin, this condition is known as Type 1 diabetes. Attacks from the body’s own immune system destroy beta cells, thereby reducing insulin secretion. People with Type 1 diabetes must take insulin to live. Five to 10 percent of people with diabetes have Type 1 diabetes, which usually develops in childhood. Type 1 diabetes is more prevalent in whites and rarely develops in people of other races.
Researchers have found that Type 1 diabetes develops because of genetic and environmental factors. Up to 50 percent of people with Type 1 diabetes have the disease because of genetic susceptibility—they inherited an increased likelihood of developing it. Mutations in a number of genes that encode proteins involved in the immune system play a significant role in the development of diabetes. Scientists believe that environmental triggers like viral infections, dietary factors, environmental toxins, psychological stress, and even season of the year can precipitate Type 1 diabetes. However, no single trigger appears responsible.
Type 2 diabetes, the number one cause of kidney failure, accounts for most cases of diabetes and is clearly linked to obesity. According to the National Institutes of Health, almost 80 percent of people with Type 2 diabetes are overweight.2 In Type 2 diabetes, although the pancreas secretes plenty of insulin, the cells of the body become resistant to it, preventing the transport of glucose into the cells. Like people with Type 1 diabetes, people with Type 2 diabetes also may need insulin supplementation in order to live. Mild cases of Type 2 diabetes can be controlled through diet and oral, non-insulin medications.
Type 2 diabetes is much more prevalent in minority populations, largely because these groups have higher rates of obesity. Native Americans have one of the highest rates of Type 2 diabetes in the world. Other minority groups greatly affected by Type 2 diabetes include African Americans, non-Hispanic African Americans, and Hispanic Americans. Because of the high rates of obesity in these populations, the U.S. Centers for Disease Control and Prevention expects the rates of diabetes to increase in the future.
Diabetes can create many complications affecting almost every part of the body. In addition to kidney failure, diabetes can lead to heart and blood vessel disease, strokes, blindness, limb amputations, and nerve damage. Babies born to women with uncontrolled diabetes can have birth defects. This is all a big price to pay for a disease that is preventable in most cases by maintaining a normal weight. Medical researchers are working hard to identify the hormonal and environmental causes of increasing rates of obesity, and to develop treatments and programs to conquer the obesity epidemic. (See below for more information about hormones and obesity and about obesity and diabetes.)
Type 2 diabetes has a poorly understood genetic component. Research is under way to determine which genes are involved and to what extent they play a role in the development of the disease. Having answers to these questions will help doctors identify who is most susceptible to diabetes and locate potential targets for treatment.
Type 2 diabetes runs in families. However, the genetic basis of the mutations that lead to diabetes varies among family members. Thus, a number of different genes may be responsible for an increased susceptibility to Type 2 diabetes. Although researchers have studied so-called polygenetic diseases for more than twenty years, they have learned that finding the genes that contribute the most to a disease is quite difficult. Type 2 diabetes is no exception, especially considering the importance of environment and lifestyle in the disease. This does not mean that scientific research has yielded no new information about the genetic contribution to diabetes. Quite the contrary! Researchers have made a good start in identifying the genes involved.
Recently, three international genetic studies examined the genes involved in insulin secretion from pancreatic cells as well as how insulin acts on cells in the body.3 These studies found at least ten genetic variants in diabetic populations, each one of which contributes small amounts to the predisposition for the disease. It is not known whether any of these variants suggest a novel approach to treating Type 2 diabetes. More extensive research is required.
Understanding the relationship between obesity and Type 2 diabetes is critical. The key to this relationship are the cells in the body and, interestingly, in the brain. Over the past decade, researchers have learned a great deal about the variety of substances that control appetite, including hormones.4
Hormones act on receptors to exert their functions. One of these functions is appetite. Receptors are specialized entities on surfaces of cell membranes that act specifically for only one hormone, similarly structured hormones, or synthetic compounds. Think of the hormone-receptor interaction as a key and a lock. Only one key (or keys very similar to it) will unlock the door so the hormone will respond appropriately.
One such hormone, called leptin, regulates appetite through an interaction with a receptor. Researchers have found that when blood leptin levels are high, we eat less, and when they are low, we eat more. When we eat, leptin levels increase. The ability of this hormone to tell us when we are full depends on its action on certain receptors, however. If these receptors do not respond appropriately to leptin, a person can eat more food despite being full, and obesity can result. Obese people often have higher leptin levels, which correlate with insulin resistance. The receptors may respond less to a given amount of leptin, and the body then secretes more of it. Exactly how changes in leptin levels, other proteins, and even inflammatory responses cause or relate to insulin resistance and diabetes is not known. However, the study of leptin levels is a promising area for future diabetes research.5
Another factor in insulin resistance resides in the brain. The brain regulates food intake by responding to levels of insulin and leptin in the blood, as well as to glucose and certain types of fat called free fatty acids. When the brain detects that the actions of these hormones are sufficient, it tells the rest of the body that it needs to consume less food. Conversely, when these signals are in short supply, the brain promotes increased food consumption. If the brain can no longer keep food intake within normal limits, weight gain and insulin resistance can result. How does this happen?
It turns out that the brain has insulin receptors that can become resistant to insulin, just as insulin receptors in peripheral tissues can. In fact, the biochemical pathways that mediate the actions of insulin appear to be similar in both the brain and peripheral tissues. Like peripheral tissues, the brain receptors become more resistant to insulin with excess food consumption. Thus, the brain’s control of food intake is impaired, resulting in obesity. The brain responds to leptin in the same way as tissues do elsewhere in the body. How obesity can lead to Type 2 diabetes is a multifaceted process.
So, how does all this add up? When we eat, food is partially converted into glucose to feed the body’s cells and to provide the energy they need to operate. To transport glucose into the cells, the pancreas secretes insulin, thereby regulating our blood levels of glucose. If we eat too much food over a long period, the cells, including those in the brain, become resistant to the constant bombardment of too much insulin. In addition, the release of leptin to control our food intake no longer controls our appetite, and we develop resistance to leptin. Finally, when insulin production is insufficient to move glucose into cells, glucose rises to dangerous levels in the blood and can result in Type 2 diabetes.
We have seen that there are many physiological mechanisms that can lead to obesity and diabetes. Genetic defects, too, can contribute to the ultimate expression of Type 2 diabetes. When these genes have been identified, it is likely that medications can be developed that will target the expression of these genes, potentially controlling excessive food intake.
How does diabetes lead to kidney failure? The process, technically known as diabetic nephropathy, typically develops over a period of ten to twenty-five years. It starts when excess glucose in the blood degrades the filtering capacity of the glomerulus in the kidney (see chapter 2). Normally, the glomerulus will allow only small molecules, like water and salts, to pass through, leaving behind large molecules like proteins. Small amounts of protein excreted in the urine, a condition called microalbuminuria, is often the first sign of diabetes. (A sign is something a doctor can identify through testing. A symptom is something the patient experiences or notices.) Kidney function is generally normal at this stage. However, if the deterioration of the filtration of blood through the glomerulus continues, increasing amounts of protein pass into the urine. Called macroalbuminuria, this excess excretion of protein can cause scarring of the glomerulus and can lead to declining kidney function.
How high glucose concentrations degrade kidney function is not completely understood. Nevertheless, researchers have discovered a number of potential biochemical pathways that are stimulated by glucose.6 These pathways seem to underlie the growth of some cells in the glomerulus and tubules that lead to scarring and fiber-like tissue, thereby degrading kidney function. If these processes continue long enough, kidney failure can result.
Hypertension, or high blood pressure, is the second leading cause of kidney failure, accounting for 24 percent of cases. Blood pressure that is too high can damage kidneys and cause them to ultimately fail. (High blood pressure is defined below.)
When the heart pumps blood through the blood vessels, the blood pushing against the walls of these vessels increases the pressure. Two numbers express this pressure: one is pressure when the heart has contracted (known as maximum, or systolic, pressure), and the other is the pressure after the heart relaxes (known as minimum, or diastolic, pressure).
Blood pressure can be measured using a blood pressure monitor. A blood pressure cuff is wrapped around the upper arm. The cuff is inflated using a pump until the pulse in the upper arm is no longer felt. The cuff is slowly deflated until the sounds of heartbeats are heard. The pressure at which the sounds are first heard is the systolic pressure, whereas the pressure at which sounds are no longer detectable is the diastolic pressure. The blood pressure monitor provides a readout of two numbers. The measured blood pressure is expressed as a ratio of these two numbers, like 120/80 (systolic/diastolic, or “systolic over diastolic”).
A blood pressure at or below 120/80 is considered normal. Higher values may be evidence of high blood pressure, or hypertension. Hypertension is categorized as more or less severe based on the degree of pressure elevation. According to the National Institutes of Health, if the systolic pressure reaches 120 to 139 or the diastolic pressure reaches 80 to 89, a person is pre-hypertensive. People with systolic pressures of 140 and above, or diastolic pressures of 90 or above, are considered hypertensive. In either case, medications and/or lifestyle changes are necessary to control blood pressure.
As we see in figure 3.3, two factors contribute to the development of hypertension: the amount of blood flowing through vessels and the vessels’ diameter. These factors affect either the systolic or the diastolic pressures. The amount of blood flowing through blood vessels results from the volume of blood leaving the heart with each contraction and the heart rate. The higher the volume of blood expelled or the higher the heart rate, the higher the systolic blood pressure. The diameter of the blood vessels affects blood pressure by resisting blood flow. The smaller the diameter of the vessels, the higher the diastolic blood pressure. This is analogous to putting your thumb over the tip of a hose with running water. Constricting the hose nozzle with your thumb, you can feel the water pressure build. One reason that vessels may be small is because of blockages due to atherosclerosis (a buildup of plaque within the vessels). There are other reasons, like other underlying medical problems, and appropriate medical treatment is determined by the cause of hypertension (see chapter 5).
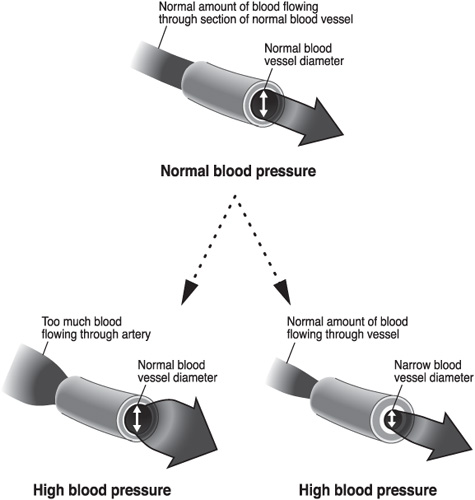
Figure 3.3. Factors in High Blood Pressure
Blood pressure is not just influenced by physiological factors like varying heart rates and the muscular tone of blood vessels. Genetic susceptibility may also influence a person’s blood pressure. Just as in diabetes research, determining which specific genes are responsible for blood pressure and their relative contribution to hypertension has been very difficult.7 Researchers do not expect that a mutation in a single gene is responsible for hypertension. Most likely, the interplay of many genes promotes hypertension. Environment and lifestyle can also contribute to hypertension through a complex gene-environment interaction. One example of this type of interaction is excessive salt (sodium) intake and retention.
Excess salt intake can lead to hypertension. However, each person responds to salt differently. At the extremes, some people are very sensitive to salt, whereas some are insensitive. These differences suggest that salt sensitivity has a genetic component. As we learned in chapter 2, the renin-angiotensin system evolved to combat dehydration by retaining sodium and maintaining blood pressure when the body loses sodium. In this system alone, it is possible that many genes could be altered in a way that promotes hypertension.
Research on genes underlying hypertension is still in its infancy and will require many more studies to identify the specific genes responsible. Future research may also yield new medications to reduce hypertension. Because hypertension plays such an important role in kidney disease, these new medications may give physicians better tools to prevent kidney failure.
Obesity contributes to hypertension as well as kidney damage induced by other diseases discussed in this chapter. Kidney diseases themselves are associated with increased blood pressure by a variety of mechanisms. Obese people often take in an excessive amount of salt, which can lead to hypertension. The high pressure on the glomerulus can slowly degrade its filtering capacity and precipitate reactions similar to those that occur in diabetes-induced kidney failure. In addition, accumulating fat can contribute to hypertension. Considering that obesity, hypertension, and diabetes often accompany one another, it is hard to know which problem came first. However, obesity is often the primary cause of hypertension and diabetes.
We know that hypertension slowly destroys the kidneys’ ability to filter the blood, but how does hypertension lead to kidney failure? With prolonged hypertension, the excess pressure can injure small blood vessels in the kidney and can destroy the filtering ability of the glomerulus (see chapter 2), leading to kidney failure. Using the hose metaphor, if you attach cheesecloth tightly over the end of the hose, water will flow through the cheesecloth without harming it. But if you pinch the hose, increasing the flow pressure, the cheesecloth will begin to degrade and eventually rupture.
Glomerular diseases are a complex set of disorders and are the third leading cause of kidney failure in the United States, accounting for 15 percent of cases. Glomerular disease often results in inflammation of the glomerulus, which can eventually cause the formation of scar tissue. As a result, protein leaks into the urine instead of being absorbed back into circulation. Like diabetes and hypertension, glomerular diseases slowly destroy the filtering ability of the glomerulus. Excess pressure on the sensitive glomerulus can lead to kidney failure. The three main causes of glomerular diseases are autoimmune diseases, hereditary nephritis, and infections.
The body’s immune system provides the first line of defense against infections by generating antibodies and immunoglobulins. However, there are times when antibodies and immunoglobulins cause harm to the body, which can lead to a number of medical problems. One of these complications is the deposit of antibodies in the glomeruli, causing inflammation.
Many autoimmune disorders contribute to glomerular diseases. One of these diseases is immunoglobulin A (IgA) nephropathy. IgA nephropathy is the most common cause of glomerular diseases not related to the presence of another disease. With IgA nephropathy, IgA deposits on the glomerulus, causing inflammation. IgA nephropathy affects men and women of all age groups equally. When a considerable amount of protein appears in the urine, controlling blood pressure helps manage the symptoms and may slow the rate of deterioration of kidney function.
Lupus erythematosus, another autoimmune disease, primarily involves inflammation of the skin and joints. This disease affects more women than men. When lupus erythematosus attacks the kidney, autoantibodies form or are deposited in the glomeruli and cause scarring. Drugs that suppress the immune system are generally used to treat the inflammation in the kidney.
One inherited form of glomerular diseases is Alport syndrome. Alport syndrome not only affects the kidney but may also impair vision and hearing. More men have difficulty with this disease than women, experiencing a decline in kidney function in their twenties and reaching total kidney failure by age 40.
Glomerular diseases are also caused by infections in other parts of the body. Similar to what happens in autoimmune diseases, the high number of antibodies produced to combat these infections can deposit in the kidneys and reduce kidney function. Although infections usually do not cause permanent damage, people with chronic infectious diseases like HIV/AIDS and hepatitis C have a risk of developing chronic kidney failure.
Focal segmental glomerulosclerosis is another glomerular disease that disproportionately affects African Americans. It results in scarring of the glomerulus or clustering of glomeruli in a specific segment of the kidney. Focal segmental glomerulosclerosis can be difficult to diagnose and treat. Biopsies to search for scarring in kidney tissue are the best means of a diagnosis. (A biopsy is a procedure in which a small amount of tissue is removed from the body for investigation and testing.) However, if the biopsy sample is from an unaffected area of the kidney, scarring will not be evident. Thus, repeated biopsies in different segments of the kidney are needed to confirm a diagnosis of focal segmental glomerulosclerosis.
Polycystic kidney disease (PKD), the fourth leading cause of kidney failure, accounts for about 5 percent of people with kidney failure. In PKD, small, fluid-filled cysts develop in the kidneys, sometimes even before birth. These cysts grow large enough over time to cause kidney failure. Because the cysts can grow so large, physicians used to think PKD was a form of kidney cancer.
According to the PKD Foundation, PKD affects 600,000 Americans of both genders and all ethnic groups. PKD has the strongest genetic link to a kidney disease. Although it can occur spontaneously, most people get PKD by inheriting it. In fact, PKD is one of the most life-threatening inherited diseases. Because PKD is primarily inherited, research has identified the genes and their corresponding proteins. As a result, a cure for PKD may be found in the near future.
People with PKD inherit the disease from one or both of their parents, depending on the form of the disease. PKD comes in two forms: autosomal dominant (ADPKD), the more common form of the disease, and autosomal recessive (ARPKD).
Genetically, the difference between ADPKD and ARPKD is the number of faulty copies inherited (see figures 3.4 and 3.5). People with ADPKD inherit the mutated gene from only one parent, whereas people with ARPKD inherit one mutated gene from both parents. The gene inherited in ARPKD is different from the one inherited in ADPKD.
ADPKD is very common, affecting 1 in 500 people. Because a person needs to inherit only one copy of an abnormal gene from one affected parent to get ADPKD, the chance of inheriting PKD when one parent has the defect is 50 percent. Because both parents must have the mutated gene, inheriting ARPKD is much less common, affecting 1 in 20,000 people in the general population. With ARPKD, if only one parent carries the mutated gene without having the disease himself, children will not develop ARPKD but may pass on the mutated gene to their children. A person with parents who both have a mutated ARPKD gene has only a 25 percent chance of inheriting the disease.
ADPKD and ARPKD also develop differently. The onset of ADPKD can occur at any age from the late teens to the mid-thirties, with kidney failure generally developing between the mid-forties and mid-fifties. Because the rate of cyst formation is variable, a person with ADPKD may not need dialysis until an advanced age. Sometimes ADPKD is diagnosed unexpectedly during a routine physical, where abnormal lab results suggest kidney disease. More tests would be needed for a definitive diagnosis (see below). ARPKD, in comparison, often progresses before birth. A person with ARPKD can only survive into adulthood with dialysis or transplantation. In this book I use the terms ADPKD and PKD interchangeably, since the incidence of ARPKD is so low.
Figure 3.4. Autosomal Dominant Inheritance of PKD (ADPKD)
Figure 3.5. Autosomal Recessive Inheritance of PKD (ARPKD)
During the last decade research has revealed the genetic underpinnings of PKD. Two genes, PKD1 and PKD2, account for virtually all cases of PKD. Mutations in PKD1 account for 85 percent of PKD cases. In most of the other cases of PKD, mutations in PKD2 are responsible.
Although the disease caused by each mutated gene is similar, people with PKD2 mutations tend to progress toward kidney failure later in life than people with PKD1 mutations. In addition, people with PKD2 mutations have fewer renal cysts when diagnosed and are less likely to have high blood pressure. People with PKD1 mutations experience kidney failure earlier in life because they have more cysts than people with PKD2 mutations.
Genes make proteins. PKD1 and PKD2 make the proteins polycystin-1 and polycystin-2, respectively, which play a crucial role in the growth of cysts, though we don’t yet know exactly how. Research has shown that the two proteins are attached to one another and that mutations in either gene can cause PKD. Although a mutation in the genes and improper working of the proteins are necessary for PKD to progress, other unknown factors may explain why the disease develops differently across families and even within families.
How renal cysts form and grow is currently under intense scientific investigation. Epithelial cells lining the tubules in the kidneys are actively involved in reabsorbing nutrients and water (see chapter 2). In PKD, 1 to 2 percent of epithelial cells reproduce many times and eventually form numerous cysts. These cysts fill up with fluid and eventually press against the nephrons so that no urine can pass through them. When enough nephrons are blocked, the kidneys cease to function. The cysts that form in the kidneys can become quite large (see figure 3.6). A normal kidney weighs less than a pound. A polycystic kidney can weigh up to 38 pounds and may produce a protruding abdomen.
Until recently, early diagnosis of PKD had been difficult, but now non-invasive techniques like ultrasound, computerized tomography (CT) scans, and magnetic resonance imaging (MRI) scans are used to identify renal cysts.8 Ultrasound detects ADPKD in most people by 30 years of age. However, ultrasound may miss some cases involving the PKD2 gene. CT scans produce clearer images but require exposure to radiation and contrast dyes.
Figure 3.6. A Polycystic Kidney (left) Can Be Much Larger Than a Normal Kidney (right)
Source: PKD Foundation.
A recent study found that measuring increases in kidney size (cyst volume) using MRI scans is the best method of diagnosing and following the progression of PKD, and was an excellent predictor of the loss of kidney function.9 Using MRI scans to measure kidney size is not yet part of general clinical practice, but this approach could provide more precise measurements of disease progression and of the effectiveness of medical treatments.
Like diabetes, PKD can have numerous health consequences. Kidney cysts can be quite painful and may require surgery. People with PKD may have blood in the urine, frequent kidney infections, and urinary tract infections that require hospitalization. In some cases, the kidneys must be removed.
People with PKD may also have cysts form in the liver or other organs. The cysts can become large and uncomfortable, especially in women, requiring removal of part of the liver or drainage of the fluid in the cysts.
A potentially fatal complication of PKD is the ballooning (called an aneurysm) and rupture of a major blood vessel, especially in the brain. Aneurysms occur in 5 to 10 percent of people with PKD. Where a family history of aneurysms exists, the risk of aneurysm rises to 20 percent. Modern imaging techniques can detect aneurysms by visualizing affected blood vessels, and people with PKD should be tested if they have a family history of aneurysms. If found early, aneurysms of a certain size can be repaired surgically. Small aneurysms are monitored with periodic scans.
Because genetic influences contribute to all four causes of kidney failure we have discussed, can tests identify people who are (or whose descendents are) at risk of developing kidney disease? Would a test help these people modify their lifestyle to reduce their chances of getting kidney disease or developing kidney failure? Perhaps. Some genetic tests can find the genes that cause diseases like diabetes. But because so many genes can contribute to each disease, it is not clear at this time how valuable such tests would be.
PKD is an exception because the genes for the disease have been identified. A genetic test has been developed by Athena Diagnostics (www.renaldx.com) to sequence PKD1 and PKD2 and to look for mutations. The test is effective only 70 percent of the time, however, so the results are possibly misleading. Sometimes the results give a false positive for the presence of PKD. Conversely, the results can miss actual cases of PKD. Results of these genetic tests should be confirmed by an ultrasound.
Although genetic testing has its advantages, there are disadvantages that might deter some people from being tested. One drawback of finding out you have a disease is the emotional burden that knowledge brings with it. Since there is little you can do about having PKD, other than good medical management for symptoms like hypertension and drinking a lot of fluid, what purpose would be served by knowing? A diagnosis of PKD may make it difficult to obtain medical and life insurance. If you do not have continuous employment as I did, a new employer may not provide adequate insurance. If you are unemployed, you may not be able to obtain insurance at all. Having a definitive diagnosis of PKD may not be useful until signs of kidney failure or other symptoms like hypertension or kidney cysts appear. Recently legislation to bar genetic discrimination in obtaining medical insurance and employment was signed into federal law, the Genetic Information Non-Discrimination Act of 2008. Currently it is not clear how well the law will be enforced or whether it will face legal challenges. In addition, Congress passed the Patient Protection and Affordable Care Act in 2010 that prohibits excluding people with preexisting conditions from obtaining health insurance by 2014.
People with PKD may have trouble deciding whether to have children. Although children have a 50-50 chance of not inheriting a PKD mutated gene from an affected parent, they also have a 50-50 chance of inheriting the disease. These odds are high enough to give some people pause before deciding to start a family. Parents can take solace in the hope that a cure might emerge for their children with PKD. In my own family, my mother died prematurely at age 44, when dialysis and transplantation were not available. Now in my sixties, I have survived with a successful kidney transplant and am living a normal life. Today’s medicine can treat kidney failure, anemia, high blood pressure, and other complicating factors of the disease. In my mother’s day, none of these treatments existed. Tomorrow’s medicine may be much more advanced, and a treatment to prevent cyst formation or growth may be available, relieving our children of the burden of PKD.
There is hope for better treatment options in the future.