Halogenated Aromatic Hydrocarbons
Abstract
In Chapter 4, we discussed the organochlorine pesticides which are one of the highly persistent organic pollutants (POPs). This chapter continues our examination of POPs by focusing on a few groups of halogenated chemicals structurally based on two benzene or phenyl rings. These include polychlorinated biphenyls (PCBs), dioxins, furans, polybrominated diphenyl ethers (PBDEs), and polybrominated biphenyls (PBBs). Halogenated means that all of these chemicals have one or more halogen atoms attached to their benzene rings. Halogens are the five elements that fall in group 17 of the periodic chart of elements and require one electron to complete their outer valence shells. All are toxic, form acids when combined with hydrogen, and form a bond with carbon that is difficult to break. The halogens we are most interested in include chlorine (Cl),bromine (Br), and fluorine (F). PCBs, dioxins, and furans have had a long and often intertwined history in manufacturing, research, and concern; thus, we will examine them first and then discuss their brominated counterparts.
Keywords
Halogenated; dioxin; furan; polychlorinated biphenyl; polybrominated diphenyl ether (PBDE); polybrominated biphenyl (PBB); cytochrome
Introduction
In Chapter 4, we discussed the organochlorine pesticides which are one of the highly persistent organic pollutants (POPs). This chapter continues our examination of POPs by focusing on a few groups of halogenated chemicals structurally based on two benzene or phenyl rings. These include polychlorinated biphenyls (PCBs), dioxins, furans, polybrominated diphenyl ethers (PBDEs), and polybrominated biphenyls (PBBs). Halogenated means that all of these chemicals have one or more halogen atoms attached to their benzene rings. Halogens are the five elements that fall in group 17 of the periodic chart of elements and require one electron to complete their outer valence shells. All are toxic, form acids when combined with hydrogen, and form a bond with carbon that is difficult to break. The halogens we are most interested in include chlorine (Cl), bromine (Br), and fluorine (F). PCBs, dioxins, and furans have had a long and often intertwined history in manufacturing, research, and concern; thus, we will examine them first and then discuss their brominated counterparts.
Introduction to Polychlorinated Biphenyls, Dioxins and Furans
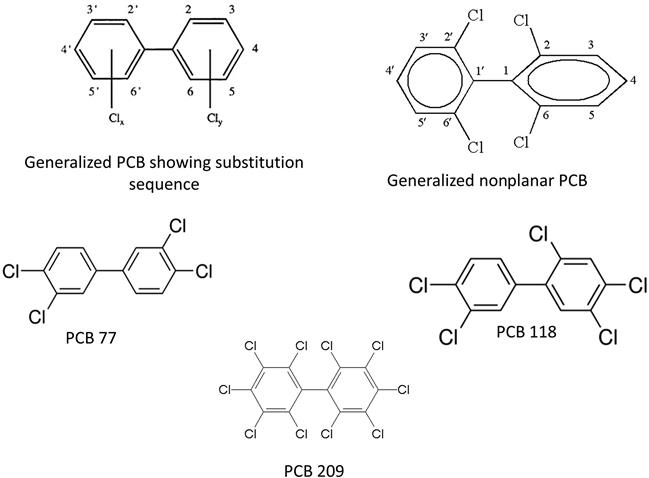
PCBs are organic molecules composed of two phenyl (hence biphenyl) or benzene rings with 1–10 chlorine atoms attached. Their general molecular formula is C12H10−xClx where x is the number of chlorines (Fig. 6.1). For each chlorine substitution, a hydrogen atom is removed. There is a maximum of 209 different individual PCBs or congeners because the number of sites where chlorine can attach is limited. PCBs are entirely synthetic. Up until the 1970s, they were used for several industrial purposes, mostly associated with cooling transformers and similar devices. Like chlorinated pesticides, PCBs are lipophilic and can both bioaccumulate and bioconcentrate. The lipophilicity and persistence are closely related to the number of chlorines in the molecule. Some PCBs cause cancer, endocrine disruption, neurotoxicity, and immunotoxicity in animals and are proven carcinogens in humans.
A small group of PCBs are termed dioxin-like because they behave like dioxins in producing harmful physiological effects. Because of their environmental persistence and suspected human carcinogenicity, PCB production in the United States and several other countries was banned in 1972 and usage was halted in 1979. PCBs were among the POPs recommended to be banned throughout most of the world by the Stockholm Convention in 2001.
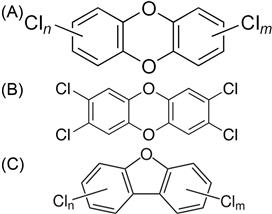
Dioxins and furans are structurally very similar to PCBs. Dioxins, more formally known as polychlorinated dibenzo-p-dioxins (PCDDs), are chlorinated organic molecules composed of two benzene rings connected to each other via two intermediary bonds with oxygen instead of directly bonded to each other as in PCBs (Fig. 6.2). Due to chlorine substitutions, there are up to 75 PCDDs with seven of these being highly toxic. Furans, also called polychlorinated dibenzofurans (PCDFs) are similar to PCDDs and PCBs because they have two benzene rings, but these are connected by a single oxygen (Fig. 6.2). There are 135 congeners of furans, 10 of which have highly toxic dioxin-like properties.
Both dioxins and furans come from several sources that typically involve high heat conditions. Volcanoes, forest fires, brush fires, the normal processing of sugar cane through burning prior to harvesting, and burning of other biomass are major natural sources of these compounds. Industrial combustion processes also produce substantial dioxins and furans.
Chemical Characteristics of PCBs, Dioxins, and Furans
On each ring of a biphenyl there are potentially five sequentially numbered reactive sites (site # 1 is occupied by the bond connecting the rings in PCBs, Fig. 6.1). If no chlorination occurs, the molecule is simply called biphenyl and the free bonds are occupied by hydrogen atoms. In PCBs, up to 10 chlorine atoms can be “substituted” onto the biphenyl, one at each reactive site. The specific congener is given a chemical name based on the number and location of its chlorine atoms. For example, 2,3′,4,4′,5-pentachlorobiphenyl is a PCB with five chlorines (hence the prefix penta-) located at sites 2,4, and 5 on the one benzene ring and at sites 3 and 4 on the other ring. Each congener can be placed in a class based on the number of chlorine ions and given a unique number so 2,3′,4,4′,5-pentachlorobiphenyl is a pentachlorobiphenyl identified as PCB 118 (Table 6.1). PCB 209 is the only PCB that is fully substituted by chlorine atoms. The extra bonding between the phenyl groups on dioxins and furans reduces the number of potential congeners, but the naming scheme remains the same as for PCBs.
Table 6.1
Some Characteristics of Selected PCBs, Dioxins, and Furans
| PCB # | Structure | CAS | Log Kowa | MWb | Solubilityug/Lc | MP °C | Vapor Pressure (mm Hg) | Half-Life (Years) | ||
| Air | Water | Sediment | ||||||||
| PCBs | ||||||||||
| Monochlorobiphenyls (N = 3) | ||||||||||
| 1 | 2 | 2051-61-8 | 4.60 | 188 | 5.5 | 35 | 1.11 | 0.01 | 0.17 | 0.6 |
| 3 | 4 | 2051-62-9 | 4.40 | 188 | 1.2 | 79 | 4.96 E-1 | 0.02 | 0.33 | 0.6 |
| Dichlorobiphenyls (N = 12) | ||||||||||
| 7 | 2,4 | 33284-50-3 | 5.15 | 223 | 1.25 | 25 | 1.61 E-1 | 0.02 | 0.25 | 0.80 |
| 10 | 2,6 | 33146-45-1 | 5.31 | 223 | 1.4 | 36 | 3.25 E-1 | 0.01 | 0.17 | 0.80 |
| 15 | 4,4′ | 2050-68-2 | 5.33 | 223 | 0.06 | 150 | 8.19 E-2 | 0.02 | 0.34 | 1.45 |
| Trichlorobiphenyls (N = 24) | ||||||||||
| 18 | 2,2′,5 | 37680-65-2 | 5.55 | 257 | 0.4 | 45 | 9.14 E-2 | 0.06 | 0.89 | 2.13 |
| 28 | 2,4,4′ | 7012-37-5 | 5.69 | 257 | 0.16 | 58 | 2.27 E-2 | 006 | 0.89 | 2.62 |
| 37 | 3,4,4′ | 38444-90-5 | 4.94 | 257 | 0.015 | 88 | 1.81 E-2 | 0.09 | 1.27 | 2.62 |
| Tetrachlorobiphenyls (N = 42) | ||||||||||
| 47 | 2,2′,4,4′ | 2437-79-8 | 6.29 | 292 | 0.09 | 84 | 9.59 E-3 | 0.17 | 2.49 | 5.71 |
| 66 | 2,3′,4,4′ | 32598-10-0 | 5.45 | 292 | 0.04 | 125 | 6.58 E-3 | 0.29 | 3.29 | 5.71 |
| 77 | 3,3′,4,4′ | 32598-13-3 | 6.52 | 292 | 0.001 | 181 | 4.92 E-3 | 0.29 | 4.01 | 5.71 |
| Pentachlorobiphenyls (N = 46) | ||||||||||
| 87 | 2,2′,3,4,5′ | 38380-02-8 | 6.37 | 326 | 0.004 | 115 | 2.72 E-3 | 0.22 | 3.09 | 4.17 |
| 101 | 2,2′,4,5,5′ | 37680-73-2 | 7.07 | 326 | 0.01 | 77 | 3.28 E-3 | 0.22 | 3.12 | 5.11 |
| 116 | 2,3,4,5,6 | 18259-05-7 | 6.30 | 326 | 0.008 | 125 | 4.08 E-3 | 0.22 | 2.97 | 2.28 |
| Hexachlorobiphenyls (N = 42) | ||||||||||
| 134 | 2,2′,3,3′,5,6 | 52704-70-8 | 7.30 | 360 | 0.0004 | 101 | 1.32 E-3 | 0.66 | 9.03 | 6.65 |
| 153 | 2,2′,4,4′,5,5′ | 35065-27-1 | 7.75 | 360 | 0.001 | 104 | 5.66 E-4 | 0.66 | 9.21 | 8.56 |
| 155 | 2,2′,4,4′,6,6′ | 33979-03-2 | 7.12 | 360 | 0.0007 | 115 | 1.57 E-3 | 0.34 | 4.93 | 8.56 |
| Heptachlorobiphenyls (N = 24) | ||||||||||
| 171 | 2.2′,3,3′,4,4′,6 | 52663-71-5 | 6.70 | 395 | 0.002 | 123 | 1.92 E-4 | 0.54 | 7.65 | 8.56 |
| 185 | 2,2′,3,4,5,5′,6 | 52712-05-7 | 7.93 | 395 | 0.00045 | 150 | 3.47 E-4 | 0.44 | 6.31 | 8.56 |
| Octachlorobiphenyls (N = 12) | ||||||||||
| 194 | 2,2′,3,3′,4,4′,5,5′ | 35694-08-7 | 8.68 | 429 | 0.0002 | 160 | 4.74 E-5 | 2.97 | 3.83 | 18.3 |
| 202 | 2,2′,3,3′,5,5′,6,6′ | 2136-99-4 | 8.42 | 429 | 0.0003 | 163 | 1.88 E-4 | 1.60 | 22.0 | 18.3 |
| Nonachlorobiphenyls (N = 3) | ||||||||||
| 206 | 2,2′3,3′,4,4′,5,5′6 | 40186-72-9 | 9.14 | 464 | 0.00011 | 207 | 1.83 E-5 | 3.25 | 41.5 | 20.5 |
| 208 | 2,2′,3,3′,4,5,5′6,6′ | 52663-77-1 | 8.16 | 464 | 1 E-5 | 184 | 3.76 E-5 | 1.25 | 17.7 | 20.5 |
| Decachlorobiphenyls (N = 1) | ||||||||||
| 209 | 2,2′3,3′,4,4′,5,5′,6,6′ | 2051-24-3 | 9.60 | 498 | 1.2 E-6 | 226 | 9.77 E-6 | 5.7 | 68.5 | 22.8 |
| Dioxins (xxx-dibenzo-p-dioxin)d | ||||||||||
| 1-chloro | 39227-53-7 | 4.75 | 218 | 0.417 | 123 | 9 E-5 | ||||
| 2,3-dichloro | 29446-15-9 | 5.60 | 253 | 0.0149 | 164 | 2.9 E-6 | ||||
| 1,2,3,4-tetra | 30746-58-8 | 6.6 | 322 | 0.00047 | 190 | 4.8 E-8 | ||||
| 1,2,3,4,7-penta | 39227-28-6 | 8.64 | 356 | 0.00012 | 196 | 6.6 E-10 | ||||
| 1,2,3,4,7,8-hexa | 29227-25-6 | 9.19 | 390 | 0.000004 | 273 | 3.8 E-11 | ||||
| 1,2,3,4,6,7,8-hepta | 35822-46-9 | 9.69 | 425 | 0.000002 | 265 | 5.6 E-12 | ||||
| octachloro | 3268-87-9 | 10.07 | 460 | 0.00000007 | 332 | 8.2 E-13 | ||||
| Furans (xxx-chlorodibenzofuran) | ||||||||||
| 2,3,7,8-tetra | 51201-31-9 | 5.82 | 306 | 0.00042 | 219 | 9.21 E-7 | ||||
| 2,3,4,7,8-penta | 57117-31-4 | 6.92 | 340 | 0.00024 | 225 | 1.63 E-7 | ||||
| 1,2,3,6,7,8-hexa | 57117-44-9 | NA | 375 | 0.000008 | 225 | 6.07 E-8 | ||||
| 1,2,3,4,6,7,8-hepta | 67562-39-4 | 7.92 | 409 | 0.000014 | 236 | 1.68 E-8 | ||||
| 1,2,3,4,6,7,8,9-octo | 39001-02-0 | 8.20 | 444 | 0.0000012 | 259 | NA | ||||

PCBs can be either coplanar or nonplanar. Imagine the two benzene rings in space. If the surfaces of the two rings are in the same plane, that is, a 3D representation would have both lie on a sheet of paper, they are coplanar (sometimes simply called planar). Substitutions at the 2 (or 2′) or 6 (6′) positions are called ortho substitutions. Ortho substitutions can produce torsion which rotates the benzene rings out of being coplanar and they become nonplanar. Using our previous explanation, one ring could lie flat on a piece of paper, but the other would pierce the axis of the paper. PCBs that do not have any chlorine atoms attached at these sites are referred to as nonortho substituted PCBs. If only one chlorine is attached at any of these sites the PCB is described as being mono-ortho substituted. Nonortho and mono-ortho substitutions give the congeners a specific structure that allows them to react with an enzyme and mimic certain dioxins. Note that, due to the extra bonding between dioxins and furans, these molecules can only be coplanar. Coplanar PCB congeners are dioxin-like because their structure allows them to interact with a specific intracellular protein, the aryl hydrocarbon (AH) receptor. The receptor enhances DNA/RNA transcription and affects several regulatory proteins. In simplistic terms, unregulated enhancement of these proteins can really complicate cellular physiology. Thus, dioxins, furans, and dioxin-like PCBs produce many toxic effects. We’ll discuss this later in the chapter. There are four nonortho substituted PCBs and eight mono-ortho substituted PCBs that are dioxin-like and of special interest to toxicologists.
The level of chlorination has substantial effects on the chemical nature of all three groups (Table 6.1). PCBs with low chlorination are odorless, tasteless, have moderate viscosity, and a clear to pale yellow color. Highly chlorinated PCBs are more viscous and have a deep yellow color. In their pure form PCDDs and PCDFs are colorless solids. Water solubility of any of these chemicals is very low. While some variation within a group with the same number of chlorine atoms exists, there is a trend for the melting points to increase with degree of chlorination. At room temperatures, PCBs tend to be solids, but some mono- and dichlorophenyls are liquid at room temperature. Dioxins and furans have higher melting points and are solids up to or more than 185°C. PCBs often served as industrial coolants at temperatures higher than their melting points, so in use they may become liquids. Chlorination also decreases water solubility, as seen in both the log Kow values and actual solubilities. In all cases, molecules with few chlorines are moderately soluble compared with many other POPs. However, those with four or more chlorine atoms have very low solubility in water. Chlorination also affects the persistence of the congeners with greater chlorination increasing persistence.
Sources and Uses of PCBs, Dioxins, and Furans
The first PCB-like chemicals were synthesized in 1865 as byproducts from coal tar and were used as sealants in place of tar. In 1881, German chemists produced the first PCBs. Commercial production increased in 1929 when Monsanto Chemical Company, the same manufacturer that produced DDT and Agent Orange during the Vietnam War (along with Dow Chemical Company) and now specializes in genetically modified crops, purchased the rights to produce the chemicals. For more than 40 years, PCBs were widely used in industry as viscous liquids that were effective in keeping electrical transformers cool without being flammable at normal temperatures. They were also used to stabilize various additives in polyvinyl chloride (PVC) plastics and reduce fire hazards. Other uses included casting agents, treating fabrics to be fire resistant, adhesives, paints, coating railroad ties, and components of vacuum pump and hydraulic fluids.
Monsanto blended PCB congeners into commercially sold Aroclors that were distinguished by the degree of chlorination. There were 12 different Aroclors marketed between 1957 and 1971 with chlorine contents ranging from 21 to 68%. Each Aroclor was identified by a four-numbered suffix indicating the number of carbon atoms and the percent of chlorination by mass. For example, Aroclor-1250 had 12 carbons (the usual number for PCBs) and was 50% chlorine. In 1974, the Monsanto Chemical Company produced slightly more than 40 million pounds (18,000 tonnes, or metric tons) of Aroclor mixtures. From 1930 to 1977, an estimated 272,700 tonnes were produced in the United States with an additional 4,204,500 tonnes in Europe (ATSDR, 2000). Under commercial production, PCBs and Aroclors have had several brand names including Asbestol, Askarel, Pyranol, Chlorinol, Saf-T-Kuhl, and Therminol just in the United States and several other names in other countries.
Neither dioxins nor furans are intentionally manufactured except in small quantities for research purposes. Instead, they may be naturally produced through incomplete combustion of organic material during forest fires or volcanic activity. Both are unintentional byproducts of industrial, municipal, and domestic incineration and combustion processes. It is currently believed that dioxin emissions associated with anthropogenic incineration and combustion activities are the predominant environmental source. In addition, dioxins, mainly 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), may be formed during the chlorine bleaching process used by pulp and paper mills and they occur during manufacturing certain chlorinated organic chemicals, such as chlorinated phenols. 2,3,7,8-TCDD is a byproduct formed during the manufacture of 2,4,5-trichlorophenol (2,4,5-TCP) which is used to produce hexachlorophene (used to kill bacteria, such as in mouth washes).
The herbicide, 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) contaminated by 2,3,7,8-TCDD was a major component of the herbicide Agent Orange that was widely used to clear jungle growth during the Vietnam war. Thousands of soldiers and Vietnamese came in contact with Agent Orange. Great controversy arose over the effects of this herbicide. It has been repeatedly shown that children in the areas where Agent Orange was used had multiple health problems, including cleft palates, mental disabilities, hernias, extra fingers and toes, and high levels of cancers. In the 1970s, high levels of dioxin were found in the breast milk of South Vietnamese women, and in the blood of US military personnel who had served in Vietnam. The most affected zones were in mountainous areas. That physical abnormalities and diseases including cancer occurred in sprayed areas was not questioned so much as who had the responsibility. The United States argued about the causes of the maladies and how much it was to blame for them. Over the years and hundreds of court cases, the government finally took responsibility, at least for American veterans.
Small amounts of furans enter the environment from several sources. Accidental fires or breakdowns involving capacitors, transformers, and other electrical equipment (eg, fluorescent light fixtures) that contain PCBs can release furans formed by thermal degradation. PCDFs also enter into the environment from burning municipal and industrial waste. Old cars that used leaded gasoline released small amounts of PCDFs in the environment. Small amounts of PCDFs may also enter into the environment from burning coal, wood, or oil for home heating.
Persistence
Table 6.1 lists some estimated half-lives of PCBs in air, water, and sediment or soil. A few words of explanation concerning the data in the table are necessary. Direct measurement of degradation is impractical because of the long half-lives of the highly chlorinated PCBs. Paasivirta and Sinkkonen (2009) modeled hourly degradation rates for all 209 PCBs using available data and structural relationships. We converted their data to years which we felt was more meaningful but, by doing so, we had to use a fairly large correction factor (8760, the number of hours in a year) and this magnified any error in estimated values. Thus, the values are rough estimates to provide some concept of just how persistent PCBs are. There are many environmental factors, both biotic and abiotic, that affect actual half-lives in natural conditions, just like there are with any halogenated organic molecule.
As with other chemical characteristics, degree of chlorination is a very important factor when considering persistence. PCBs in air degrade most rapidly and those with only one to three chlorine atoms have half-lives measured in a few days. In contrast, the half-life of PCB 209 in air is 5–6 years. PCBs are more stable in water than in air and even more stable in soils and sediments than in water. Ultraviolet radiation effectively degrades PCBs and exposure to UV is greatest in air. Low-chlorinated PCBs in sediments have half-lives measured in months, but those with five or more chlorines have half-lives of several years.
Of these environmental sources, water and soil or sediments serve as the primary reservoirs of PCBs (Rice et al., 2003). Although PCBs are not very soluble in water, the vast amounts of salt and freshwater on the planet allow for substantial storage. Even though vapor pressure of PCBs tends to be low, volatilization from sediments and soils is the main way PCBs enter the atmosphere.
The half-life of 2,3,7,8-TCDD ranges from 9 to 15 years in surface soils, 25-100 years in subsurface soils, seven years in humans, and only 8.3 days in air (ATSDR, 1998). Furans are probably very similar, although there is less known about their persistence. As with all the other groups, various environmental factors can greatly alter the persistence. For example, while the whole body half-life in humans is around 8 days, furans can persist in water for 0.4-255 days, depending on the congener and environment (ATSDR, 1994).
Breakdown of PCBs
Despite their persistence, these molecules will break down or dechlorinate eventually. They are slowly metabolized by many living organisms, including mammals, plants, fungi, and bacteria (Tehrani and Van Aken, 2014). One method of natural reduction of PCBs is to convert them to their hydroxylated forms by adding an –OH group. However, hydroxylated PCBs are often more toxic than their parent forms and this step may actually make it more difficult to breakdown the PCB further. OH-PCBs formed by metabolic activity in living tissues can be released into the environment and enter the food chain by excretion, predation, and natural cycling of vegetation (Tehrani and Van Aken, 2014). Hydroxylation can also occur in the atmosphere through chemical binding with free –OH radicals.
The most common methods to remediate heavily contaminated soils and sediments is to mechanically remove the soils and dispose of them in hazard landfills or to cap an onsite landfill, thus containing the PCBs at the contaminated site. The technology to reduce the chlorination of the PCBs in these landfills is improving and often relies on enhancing microbial decay of the molecules. In addition to UV light, under natural conditions a variety of microorganisms will slowly metabolize PCBs, dioxins, and furans in soils or sediments. In fact, many different additives or activities to soil that promote bacterial decay can enhance the dechlorination, even with highly chlorinated congeners. Many of the PCBs in water will eventually deposit into sediments, where they will be buried and removed from contact with organisms. By turning soil over, helping to aerate it, and mixing it with organic matter, even earthworms can accelerate the degradation of PCBs.
As with chlorinated pesticides, plants can take up these contaminants from soils or sediments and then the plants can be disposed of or burnt. Plants can also be used to enhance bacterial decomposition.
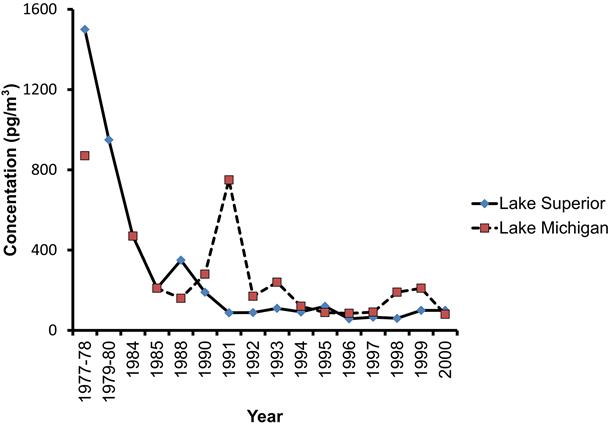
PCBs concentrations are diminishing in many environmental sources because they are no longer manufactured or used in the United States or other countries. For example, Buehler et al. (2002) reported on 23 years of monitoring atmospheric PCBs in Lake Michigan and Lake Superior. They observed a general decline in PCB concentration (Fig. 6.3) so that total PCBs dropped by approximately 91% between 1978 and 2000 along Lake Superior.
Examples of PCB Concentrations in Environmental Sources
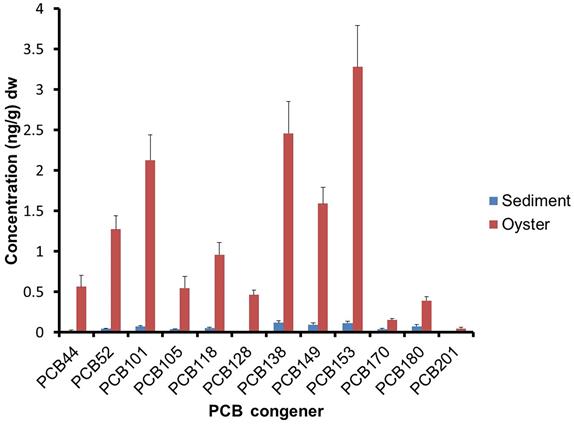
PCBs can bioconcentrate and biomagnify due to their lipophilic and persistent natures. For instance, the average and maximum congener concentration in oysters was 20 and 60 times higher than in sediments, respectively (Villeneuve et al., 2010, Fig. 6.4). Oysters may obtain some of their contaminant burdens through their food, but they undoubtedly obtain much of it directly from the sediments. Bioconcentration factors (BCF) for specific congeners ranged from 3 for PCB-170 to 60 for PCB-128. Differences in the Kow and Koc values for these PCBs were probably major factors for the differences in BCF.
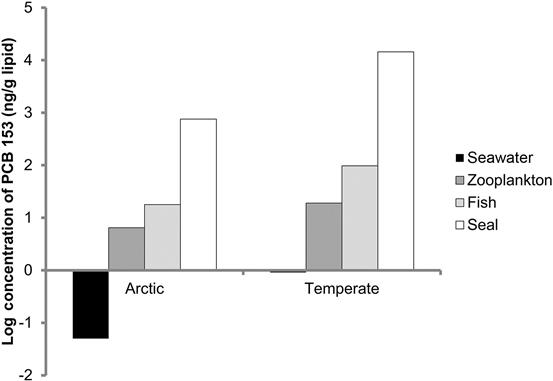
As an example of biomagnification, Sobek et al. (2010) investigated four PCBs in simplified food chains in the Arctic Barents Sea and in the more temperate environment of the Baltic Sea. In each area, PCB concentrations in water, zooplankton, fish, and seals were measured (Fig. 6.5). BCFs ranged from 18 to 288 from saltwater to zooplankton, 2.4 to 7.6 from zooplankton to fish, and 2.3 to 148 from fish to seal. The total BCFs for the Barents Sea food chain from water to seal ranged from 4022 to 15,180, whereas those Baltic Sea ranged from 218 to 21,860.
PCB concentrations are least in air, intermediate in water, and highest in sediments and soil. Hence, atmospheric concentrations of PCBs are usually expressed in pg/m3 or parts per quintillion; water concentrations are in ng/L or parts per trillion; and soil or sediment concentrations are usually in ng/g or ug/kg—both of which are parts per billion (Table 6.2). Some very contaminated sites, such as landfills, may have soil concentrations in the low mg/kg or parts per million.
Table 6.2
Some Representative Concentrations of Total PCBs in Air, Water, and Sediments or Soils
| Location | Conditions | Range | Units | Year | Source |
| Atmosphere | |||||
| Argentina | Urban | 200 ±130 | pg/m3 | 2006–2007 | Tombesi et al. (2014) |
| Argentina | Rural | 20 ±20 | pg/m3 | Tombesi et al. (2014) | |
| China | Electronic waste site | 7825–76330 | pg/m3 | 2007–2008 | Chen et al. (2014) |
| China | Rural | 24.2–3552 | pg/m3 | Chen et al. (2014) | |
| Chile | Rural | 40 | pg/m3 | 2007 | Pozo et al. (2012) |
| Chile | Urban | 160 | pg/m3 | Pozo et al. (2012) | |
| Chile | Industrial | 100–350 | pg/m3 | Pozo et al. (2012) | |
| Water | |||||
| Pakistan | River Chenab | 0.21–27.5 | ng/L | 2013 | Mahmood et al. (2014) |
| China | Yangtze River | 2.02–15.67 | pg/L | 2010 | Zhang et al. (2014) |
| France | Sewage sludge | 0.28–0.70 | ng/g | 1999–2000 | Blanchard et al. (2004) |
| Sediments | |||||
| Pakistan | River Chenab | 0.83–59.4 | ng/g | 2013 | Mahmood et al. (2014) |
| Wisconsin | Fox River | 0.2–17.6 | ng/g | 1999 | Imamoglu and Christensen (2004) |
| Sweden | Boat yards | 3.0 ±5.7 | ng/g dw | 2000–2011 | Eklund and Eklund (2014) |
| United States | Indiana Harbor | 12,570 | ng/g dw | Liang et al. (2014) | |
| United States | Lake Erie | 1.9–245 | ng/g | 1997–2000 | Marvin et al. (2004) |
| Lake Ontario | 2.6–255 | ng/g | 1997–2000 | Marvin et al. (2004) | |
| Lake Michigan | 220 | ng/g | 1997–2000 | Marvin et al. (2004) | |
| Soil | |||||
| China | Solid waste incinerator | 413 ±298 | pg/g | NA | Zhang et al. (2014) |
| Chemical plant | 229 ±299 | pg/g | NA | Zhang et al. (2014) | |
| Power plant | 314 ±298 | pg/g | NA | Zhang et al. (2014) | |
| Tibet | Rural | 0.22–2.71 | ng/g | 2011 | |

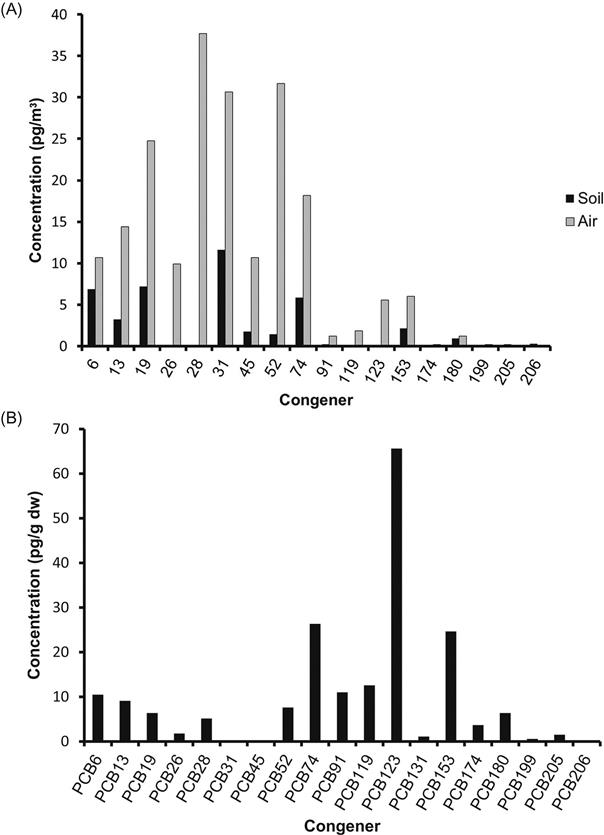
The composition and concentration of PCBs in an environmental source will depend on the source of the PCBs and the Aroclors used in that source, the amount of dechlorination that has occurred, atmospheric deposition of other PCBs, and the type of environmental source. Of the 209 possible polychlorinated biphenyls, only 130 were manufactured commercially (UNEP, 1999). Although the PCB-containing Aroclors that were sold on the market varied in their composition from low to high chlorinated PCBs, most contained at least some of the moderately chlorinated PCBs, from PCB-70 to PCB-140, and these are among the most common congeners found. PCBs with fewer chlorines tend to be more volatile than those with six or more chlorines and tend to be more common in the atmosphere. Soils and sediments tend to reflect what was used in the region, but older sediments or soils may show some bias towards highly chlorinated PCBs which are less volatile and more persistent. Compare Fig. 6.6B which is a congener profile from Turkish soils to Fig. 6.6A from air above those soils and you’ll see that the patterns are not the same. The air profile has predominately low to moderately chlorinated congeners while the soils present a more complete profile of low, moderate, and highly chlorinated congeners.
Concentrations of PCBs in Some Animal Tissues
Eisler (2000) conducted an extensive review of PCB concentrations and effects in organisms up to that time. Changes in PCB concentrations are slow and, if anything, may be lower because PCBs are no longer used—although they are very persistent. We’ll use his tables for reference. Concentrations of specific congeners vary predictably by characteristics of the congener, species of concern, and location. As with environmental samples, very low molecular weight congeners will volatize, which may make them unavailable to most organisms. The highest molecular weight congeners are mostly in the soil and sediments and not very mobile so they are less common in organisms. However, the heaviest congeners, if they become assimilated, are also the most persistent and tend to remain in organisms for many years. Generally, middle-weight congeners will most commonly be found in animals and plants. Plants may assimilate PCBs, but the greater concern is animals. Animals with relatively high concentrations of body lipids will tend to have more PCBs stored in their tissues than lean animals. Body concentrations are frequently lipid-adjusted or based on percent or total lipids because of the affinity of PCBs (and the other contaminants described in this chapter) to lipids. It is common sense to suspect that animals collected from contaminated sites will have higher concentrations than those from reference areas.
Marine mammals are the most vulnerable and most likely target of PCBs (Tanabe, 1988; Eisler, 2000). They contain a lot of fat in the form of insulating blubber. In addition, the metabolic potential to degrade organochlorine contaminants is lower in cetaceans than in many terrestrial animals (Tanabe et al., 1987; Kannan et al., 1993). Therefore, we often see lipid-corrected PCB concentrations in these animals at the mg/kg range rather than in the μg/kg range. For example, concentrations in the striped dolphin (Stenella coeruleoalba) from the Mediterranean Sea in the 1990s were among the highest reported in the literature (Eisler, 2000). Total PCB concentrations in lipids have been recorded as high as 855 mg/kg and other studies have reported 480 mg/kg fresh weight. Specific congeners ranged from 0.043 to 47 mg/kg lipid weight. Compounding the high overall concentration, approximately 53% of the total PCBs were composed of coplanar congeners (Kannan et al., 1993) with dioxin-like properties that have substantially higher toxicities than other PCBs. The dolphins in that study were experiencing a viral epizootic and it was suspected that the high concentrations of PCBs may have compromised their immune system. In contrast to striped dolphins, studies on other cetaceans and pinnipeds usually report total PCB concentrations in the μg/kg range to 50 mg/kg lipid weight, but killer whales or orca (Orcinus orca), at the top of the marine food chain, have been reported with more than 370 mg/kg (Tanabe et al., 1987).
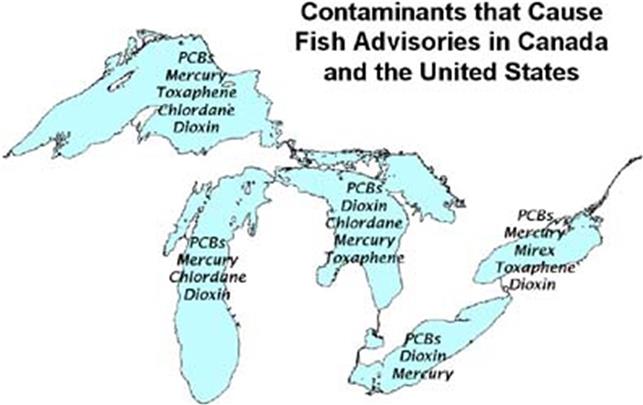
Some fatty fish have fairly high concentrations of PCBs, depending on location. Flounder collected from contaminated waters had 124 to 333 mg/kg total PCBs dry weight whereas those from a reference area had only 4 to 13.3 mg/kg. Lake trout (Savelinus namayacush), a top freshwater predator, were reported to have up to 8 mg/kg lipid weight total PCBs in 1977, but this gradually declined to 2.5 mg/kg by 1988 (Borgmann and Whittle, 1991). Fish advisories warning people to limit or cease consuming fish from particular lakes due to high levels of contaminants including PCBs exist for several states in the East and Midwest (Fig. 6.7).
There are some examples that illustrate certain aspects of PCB contamination. Adult male burbots (Lota lota) in Lake Erie (Stapanian et al., 2013) had higher PCB concentrations than adult females or juveniles. Lower concentrations in females is often attributed in part to maternal transfer from mother to eggs. Likewise, juveniles have less PCBs than adults due to the accumulation of the contaminant through time. In this case, however, the authors suggested that the higher burdens in males was due to habitat partitioning—males tended to congregate in back bays of Lake Erie that had higher sediment concentrations of PCBs and other organics than the sites used by females and juveniles.
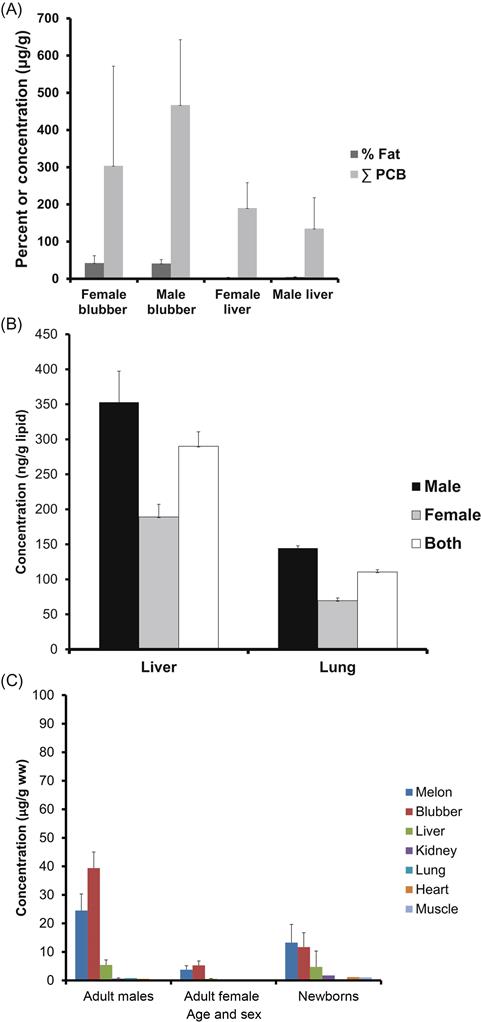
Gender and age differences in PCB concentrations seem to be common among animals. It stands to reason that as a healthy animal ages it will accumulate greater concentrations of PCBs, especially the highly chlorinated, fat soluble, and persistent congeners. Other examples of gender differences may be seen in Fig. 6.8A–C. Adult California sea lion males (Zalophus californianus) are around 3.5 times larger than adult females and feed on larger fish, which contributes to their higher body burdens of PCBs (Nino-Torres, Fig. 6.8A); this is in addition to the loss females can experience when pregnant and at parturition. This figure also presents some idea of the difference in PCB concentration between organs and tissues. Blubber has substantially more fat than liver does and, although both tissues are prime areas for PCB concentration, blubber has a higher PCB concentration than liver. Predictable gender and tissue or organ differences also exist in European red fox (Vulpes vulpes, Fig. 6.8B, Tomza-Marciniak et al., 2011) and among striped dolphins in the Mediterranean Sea (Fig. 6.8C, Storelli et al., 2012). Fig. 6.8C also shows variation among organs; both the liver and blubber have high concentrations of lipids.
A study on the little brown bat (Myotis lucifugus) (Kannan et al., 2010) focused on a bat colony where the fungal disease white-nose syndrome was prevalent and one where it was not present. White-nose syndrome has devastated many colonies of bats, particularly in the eastern and southern United States. An objective of the study was to determine if organic contaminants were associated with white-nose syndrome, perhaps through compromising bat immune systems. The authors found high concentrations of several halogenated contaminants in both sites and could not conclude that the contaminants were related to the presence of white-nose syndrome. In New York, in a diseased colony lipid weight concentrations of total PCBs ranged from 1900 to 35,000 μg/kg while in a Kentucky reference colony, the concentrations ranged from 17,100 to 18,500 μg/kg lipid weight. On the other hand, total PCBs ranged from 520 to 900 μg/kg lipid weight in New York and 4300 to 13,000 μg/kg in Kentucky.
Recall that there are 209 possible PCB congeners. Even with only 103 manufactured, there is remarkable potential for variation among species and among conditions in the actual congener composition that makes the total PCB burden in an animal. This variation is due in large part to location—what if any PCB Aroclors were used in the region and whatever contribution precipitation of PCB made to this mix. Variation is also due to the specific congeners themselves. Low-chlorinated PCBs that tend to be more water soluble are lost comparatively soon after acquisition, both in the environment and in the organism. Middle-weight congeners were the most widely manufactured and released PCBs when they were used and some may have formed after initial release due to dechlorination of higher weight congeners. Another factor is that many studies that examine congener-specific body burdens do not attempt to quantify the entire range of congeners.
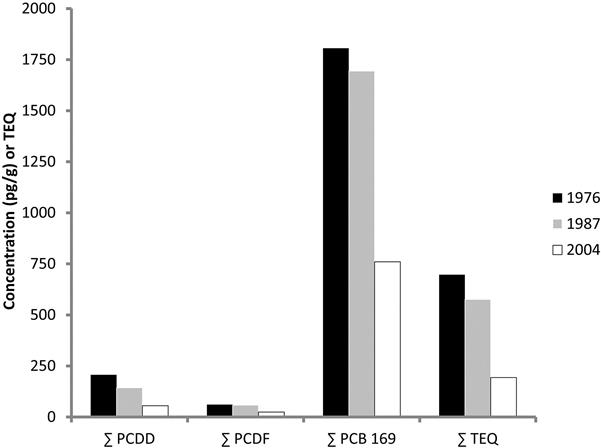
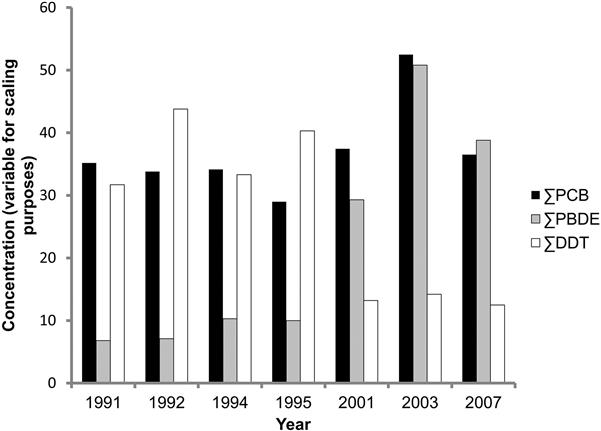
In general, the concentrations of PCBs in abiotic and biotic compartments of the environment are decreasing, but not as a universal phenomenon. Polar bears (Ursus maritimus) in the Hudson Bay region actually experienced a 149% increase in ∑ PCBs between 1991 and 2003, although this decreased to a wash by 2007 (McKinney et al., 2010). Part of the difference may have been due to sampling issues in that males have much higher PCB concentrations than females and a shift in sex ratios in sampling could make a difference, but it was obvious that the bears are still being exposed to PCBs. Among ivory-billed gulls (Pagophila eburnea) living in the Canadian Arctic, PCB concentrations dropped 60% from 1976 to 2004 and dioxins and furans decreased by approximately the same amount although these compounds occur naturally or are still used to a limited extent (Braune et al., 2007, Fig. 6.9). In contrast, however, PCB concentrations did not change between 1991 and 2007 in another polar bear population (Fig. 6.10, McKinney et al., 2010), while DDT concentrations decreased by 60% and PBDEs actually increased by 570% over the same time. Concentrations in human milk seem to be declining in parts of the world. For example, Ryan and Rawn (2014) demonstrated that PCBs in human milk in Canada have declined by 11% between 2002 and 2010. While organochlorine concentrations have decreased in parts of Spain since the 1980s, PCB concentrations are still of concern for some wildlife (Mateo et al., 2012). The air over the Great Lakes is definitely cleaner than it was in the 1970s. Total PCBs dropped 93%, and 91% over Lake Superior and Lake Michigan, respectively (Buehler et al., 2002). The Environmental Protection Agency (2015) has determined that, at least a few years ago, PCBs in Great Lakes commercial fisheries have been declining by 3–7% per year. A few studies have suggested that global climate change involving the melting of Arctic ice may release the PCBs that have been locked there for decades and result in an increase in global concentrations (eg, Bogdal et al., 2013). I guess we’ll have to wait and see.
Biological Effects of PCBs
Review the discussion on organochlorine pesticides and plants from Chapter 5 because similar relationships pertain to PCBs and plants. Generally speaking, PCBs are not thought to be toxic to plants because the more toxic, highly chlorinated PCBs including those that have dioxin-like properties are not absorbed very well by most plants. Gichner et al. (2007) found that tobacco plants (Nicotiana tabacum) grown in soils from a disposal site with heavily contaminated soil (165–265 mg/kg PCBs) had reduced growth, smaller leaves, and increased DNA damage compared with control plants. The authors did not see any increase in actual mutations, however. These effects were considered mild to moderate, especially when the elevated PCB concentrations are considered. I cannot help but think that using this tobacco for smoking would definitely increase one’s risk of health problems.
Plants have been used to help remediate contaminated soils because they can assimilate some PCBs. For instance, Viktorova et al. (2014) genetically modified tobacco plants by adding a bacterial gene to produce an enzyme that breaks the diphenyl ring. Genetically modified plants were more tolerant to PCB-contaminated soils than nonmodified plants and they were more efficient in reducing PCB concentrations in a dumpsite.
One way to remove PCBs from aquatic sediments is to dredge the sediments and place them in spoil banks on land; then phytoremediation can be used to remove PCBs. This process is being studied and questions such as ideal plant species to use under various conditions need to be more clearly understood. Plants vary widely in their ability to take up PCBs, suggesting that “weeding” out less efficient species could be beneficial. The common pumpkin (Curcurbita pepo) and other members of the squash and gourd family Curcubitaceace offer promise in efficient removal of PCBs (Zeeb et al., 2006). Cucurbits also have been demonstrated to take up and transport other soil-bound POPs, including DDE, chlordane, dioxins, and furans. Common wisdom suggested that only the more water-soluble PCBs would be taken in by plants, but pumpkin roots, sap, and shoots are unusual in that they can assimilate virtually all PCBs, even those that are highly lipophilic (Greenwood et al., 2011). BCF have been around 5.2 for roots and 1.6 for shoots. Assimilation of PCBs by plants, however, poses some risk if the PCBs are ingested. Obviously, one would not want to eat pumpkins grown on a contaminant landfill (they could still be used in jack-o-lanterns, however), but PCBs can be found in plants used for herbal teas (Amakura et al., 2009) and potentially some vegetables or forage plants affecting livestock.
In Chapter 5, we discussed the benefits of organochlorine pesticides that grizzly bears (Ursusarctos horribilis) get from eating plants (Christensen et al., 2013). PCB elimination is also enhanced by herbivory. The authors estimated that inland bears consume 341–1120 μg of PCBs daily when salmon are available. When salmon stop spawning and only plant material is available to inland grizzlies, their herbivorous diets contain a mean of 8.42 μg PCBs per day. During that time, PCB concentrations in grizzly feces may be as high as 4500 times that in their diet and it appears that grizzlies get to reduce substantial body concentrations of PCBs.
Toxicity of Dioxins, Furans, and Dioxin-Like Compounds
Dioxins are highly toxic chemicals that are linked or known to produce a range of effects in humans and other species including cancer, endocrine disruption, neurological disorders, liver toxicity, cardiovascular disorders, genotoxicity, and several other pathological conditions (ATSDR, 1998). The most toxic of the dioxins is 2,3,7,8-tetrachlorodibenzo-p-dioxin or 2,3,7,8-TCDD (TCDD for short). When people speak of dioxins, they often mean this particular congener. Animals are usually exposed through the food chain and consuming those animals as part of that food chain is how most humans become exposed to dioxins. Of great concern is that dioxins and other halogenated contaminants can be passed to young mammals and children from their mother through the placenta or from milk. In a study of 418 mothers and their children born between 1997 and 2001 in Denmark, a combination of PCDD, PCDF, and PCBs were sampled and evaluated for their combined toxic equivalency or toxic equivalency quotient (TEQ)—see next section (Wohlfahrt-Veje et al., 2014). Toxic equivalence in milk increased significantly with maternal age and fish consumption. Environmental exposure to dioxin-like chemicals was associated with babies being underweight at birth and with accelerated early childhood growth (rapid catch-up growth).
While we will cover PBDEs in more detail below, it is appropriate to include them in this discussion of maternal transfer. According to Zota et al. (2013), pregnant women at San Francisco General Hospital in 2008–2009 had the highest concentrations of PBDEs and their metabolites ever reported from pregnant women around the world. Another sample of women collected in 2011–2012 showed that concentrations of PBDEs had decreased significantly, especially in the penta- and octaPBDEs that were banned by California in 2004. Similarly, there was a modest, insignificant decline in PCB concentrations between 2008–2009 and 2011–2012. The authors concluded that PBDE exposures were likely declining due to regulatory action, but the relative stability in PCB exposures suggests that exposures may eventually plateau and persist for decades.
Other mammals show similar conditions. As one example, female polar bears demonstrated maternal transfer of PCBs and mercury through milk to their cubs (Knott et al., 2012). Total concentrations of 11 PCBs in milk ranged from 160 to 690 μg/kg ww. Although the daily intake levels for PCBs through milk consumption for cubs of the year exceeded the tolerable thresholds, calculated TEQ in milk were below adverse physiological thresholds for aquatic mammals. The authors suggested that relatively high concentrations between nondioxin-like PCBs in polar bear milk and blood could impact endocrine function of Southern Beaufort-Chukchi Sea polar bears.
Maternal transfer can also occur in egg laying animals due, in part, to the high lipid content of yolk. Transfer has been found in all sorts of animals from birds to alligators and marine turtles. One example of maternal transfer can be seen with the eggs of common snapping turtles (Bishop et al., 1995). For PCBs, the first five eggs (mean =23.9 mg/kg) had 16% more PCB than the last five eggs (mean =20.1 mg/kg).
General Mechanisms of Toxicity
Among the PCB congeners, only a few have a toxicity worth noting. These are the nonortho and mono-ortho substituted PCBs that have dioxin-like properties. There are 12 PCBs with dioxin-like properties, four nonortho substituted congeners (PCB 77, 81, 126, and 169) and eight mono-ortho substituted congeners (PCB105,114,118, 123,156,157,167, 189). The common characteristic of the highly toxic dioxins, and the furans and PCBs with dioxin-like qualities is that they can bind with the AH receptor in animals and exert their toxic effects through this bond. The relative toxicity of dioxin-like chemicals varies considerably. To make some sense of the risk from dioxin-like chemicals, the World Health Organization (WHO) developed the concept of TEQs and factors (Van den Berg et al., 2006). Each dioxin-like compound has a toxic equivalent factor (TEF) and the mixture of dioxin-like compounds is assigned a TEQ based on the sum of TEFs. The TEF is based on the toxicity of 2,3,7,8-TCDD. TCDD is given a TEF of one and the toxicity of all other chemicals are a factor of this. Thus, PCB126 has a TEF of 0.1, which suggests that its toxicity is one-tenth of TCDD. Dioxins other than TCDD, furans, and nonortho-substituted PCBs have TEFs ranging from 0.1 to 0.0003. Mono-ortho-substituted PCBs have much lower toxicity, approximately 0.00003 as toxic as TCDD.
TEFs and TEQ are not intended to be exact figures. Some of the TEFs were developed in vivo from studies with live animals, usually rats. Others were determined from less accurate studies using cell cultures. In addition, many factors, not the least of which is the subject species, can influence toxicity. The real value of these estimates is that animals and people are seldom exposed to only one dioxin-like contaminant. Several of these compounds are most often found together and a subject is exposed to all of these, each with their own toxicity. To evaluate risk, therefore, we need to place some sort of educated guess value on an exposure to these mixtures. It is fairly safe to conclude that a TEQ of five is going to be more toxic than a TEQ of 0.5, but to suggest that the first is exactly 10 times more toxic than the second is stretching the limits of the system quite a bit. It is also fair to say that the toxicity of most dioxin-like PCBs is low to very low compared to TCDD.
Polychlorinated biphenyls, especially those with dioxin-like properties, are much more likely to produce sublethal effects than acute mortality at environmentally realistic concentrations. Concentrations necessary to produce acute mortality are two to three times or higher than most environmental concentrations. However, the sublethal effects of PCBs are numerous. PCBs can cause endocrine disruption, neurotoxicity, immunotoxicity, embryonic developmental problems, teratogenicity, bronchitis, low birth weights in humans, and some genotoxicity at high concentrations. Dioxins are proven carcinogens (WHO, 2010); dioxin-like PCBs have been linked to cancer in animals and appear to produce precancerous conditions in humans. They are considered to be probable carcinogens although there has yet to be direct cause and effect relationship established. The liver is the primary organ of PCB metabolism and hepatatoxicity (liver damage), including hepatamegaly (liver enlargement) and necrosis (cell death or tissue damage) are often seen following PCB exposure in laboratory animals and humans.
Toxicity from dioxin-like PCBs is due to their interaction with the AH receptor. This receptor enhances gene transcription and is involved with many genes. TCDD and to a lesser extent, other dioxin-like compounds bind to the AH receptor. This induces the P450 1A class of enzymes that then break down toxic compounds. During the breakdown process, PCBs may be converted to their even more toxic hydroxylated forms (OH-PCB). Activation of the P450 system can be used to infer exposure to toxic compounds, but the system operates on a broad range of chemicals so exposure to PCBs specifically is not assured.
More on the Cytochrome P450 System
This is probably a good place to discuss the cytochrome P450 system more fully. Cytochromes are hemeproteins (sometimes also called hemoproteins) containing heme (iron) groups and are primarily responsible for the generation of adenosine triphosphate (ATP) via electron transport. Cytochromes consist of many families that have specific roles in respiration and photosynthesis. Cytochrome P450 or CYPs is a superfamily of hemeproteins that are typically the last of a set of oxidizing enzymes in electron transfer chains. By convention, specific enzymes in the system are referred to by CYP followed by a number such as CYP1A. The genes that code for these enzymes follow the same convention except that they are italicized. The gene CYP1A, therefore, codes for the enzyme CYP1A.
CYPs occur throughout living organisms including animals, plants, fungi, single-celled organisms, bacteria, and viruses. More than 18,000 distinct CYPs have been identified across this spectrum. In vertebrates, CYPs can be found in virtually every organ and tissue but those that are involved in the metabolism of toxic compounds such as organochlorine pesticides, PCBs, PBDEs, polycyclic aromatic hydrocarbons, and others have their highest concentrations in the liver. The primary metabolic function of these and most other CYPs is part of what is called Phase I metabolism which involves insertion of one oxygen atom from the O2 molecule into an organic substrate (eg, PCB) while the other oxygen atom combines with hydrogen to form water:
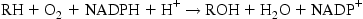
where R is the organic substrate.
The actual process is quite complex and we will only touch on the basics here. Interested students might want to consult one of the many books recently written on the topic. During this process, various molecules called radicals such as peroxide (O2−2) and superoxide (O2−1) can be generated and these radicals can cause oxidative stress in cells (Lewis, 2002). Oxidative stress affects several aspects of cellular metabolism and can cause physical damage to cellular components. Several diseases including cancer, muscular dystrophy, autoimmune diseases, emphysema, Parkinson’s disease, multiple sclerosis, atherosclerosis, and cancer have been linked to oxidative stress (Halliwell, 1987) and it is reasonable to suggest, if not already demonstrated, that it may also be a cause or at least linked to analogous diseases in animals.
One enzyme, CYP2E, is particularly linked to producing oxidative stress by accentuating the generalized P450 reaction beyond the ability of the cells to remove the harmful byproducts. Whereas CYP1A is the primary gene activated by exposure to organic contaminants, CYP2 genes may also be induced. Moreover, CYP1A can also produce its own toxic effects and further research on this is important. The bottom line is that organisms have developed a system to detoxify chemicals that they would naturally encounter. However, anthropogenic chemicals such as OCPs, PCBs PBDEs, and PAHs can either overwhelm the system or “trick” it into producing metabolites that are many times more toxic than the parent compounds.
General activation of the P450 system is not very predictive in identifying the types of contaminants an organism may be exposed to. Activation of the system and the occurrence of oxidative stress can reveal that an animal has been exposed to some contaminant and may be experiencing pathological effects, but the range of possibilities is huge. Fortunately, there are other enzymes that can be used to narrow down the field of possible contaminants affecting an individual. These enzymes are responsive to certain families of organic contaminants. They will not identify the specific congener or molecule, but they can be used as bioindicators for a group of contaminants. They have been found in most vertebrates in varying concentrations and sensitivities. Ethyoxyresorufin-O-deethylase (EROD) is induced by PAHs, coplanar PCBs, dioxins, and furans in a dose-dependent manner. The activation of EROD in tissues provides evidence for the induction of cytochrome-dependent P450 monooxygenases in the CYP1A subfamily upon exposure to contaminants. From a practical if not oversimplified perspective, while the CYP enzymes assist in the metabolism of the contaminants, EROD and its associated enzymes can be used to denote exposure. Other enzymes that can be used as biomarkers of exposure include benzyloxyresorufin-O-deethylase (BROD) and pentoxyresorufin-O-deethylase (PROD) (Heinonen et al., 1996). BROD and PROD are less sensitive to the types of contaminants that induce CYP1A enzymes and more responsive to those that induce CYPB2B or CYPB3A enzymes. More specifically, EROD and the others are separable in that EROD can be induced or activated by β-napthaflavone whereas PROD and BROD are responsive to phenobarbital. The activity of each of these enzymes can be determined by their ability to breakdown their specific substrates (eg, 7-ethyoxyresorufin for EROD). From a molecular scientist’s perspective, that difference provides a good separation between the enzymes.
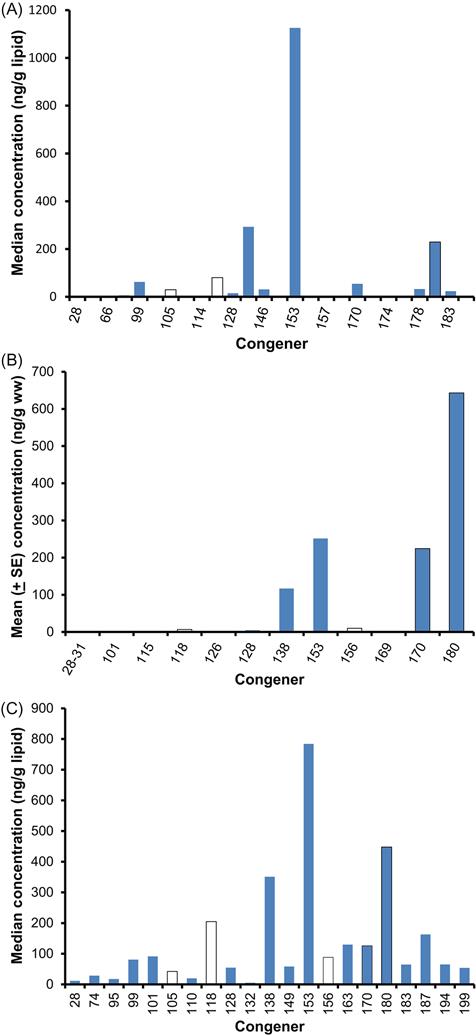
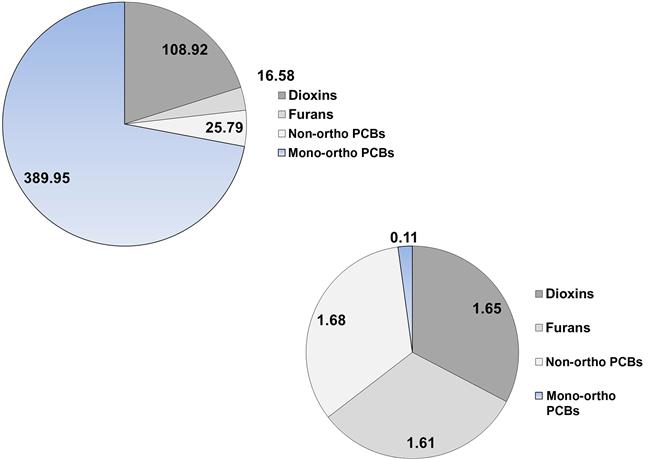
Because most of the toxicity of PCBs is due to a handful of dioxin-like coplanars, a congener profile can be misleading. For example, take a look at Fig. 6.9 that shows the congener profile of the walrus (Wolkers et al., 2006), European red fox (Mateo et al., 2012), and the great tit (Dauwe et al., 2005). These species were selected simply to show some of the variation in congener profiles. The walrus profile (Fig. 6.11A) was represented by 28 congeners for a total PCB (∑ PCB) concentration of 2000.8 μg/kg based on lipids. Of these congeners, however, PCB 153 accounted for 56% of ∑ PCB and the PCBs that had dioxin-like toxicity accounted for only 20% of the total concentration. The TEQ of this profile is 0.003 μg/kg, assuming that the TEFs are reliable for the walrus. The profile for the European red fox (Fig. 6.11B) had 14 congeners measured, but most were very low in concentration for a ∑ PCB of 1262 μg/kg. 51% of the ∑ PCB was due to congener 180 and dioxin-like PCBs amounted to 70% of the total. The TEQ was 0.017 μg/kg. The great tit had the most homogenous profile with 21 congeners measured and a ∑ PCB of 2890 μg/kg lipid weight (Fig. 6.11C). The most common congener, PCB 153, only accounted for 27% of the ∑ PCB; 31% of the ∑ PCB was in dioxin-like PCBs. The TEQ for great tits was 0.010 μg/kg. Thus, we would have to conclude that the fox was at the greatest risk of the three species.
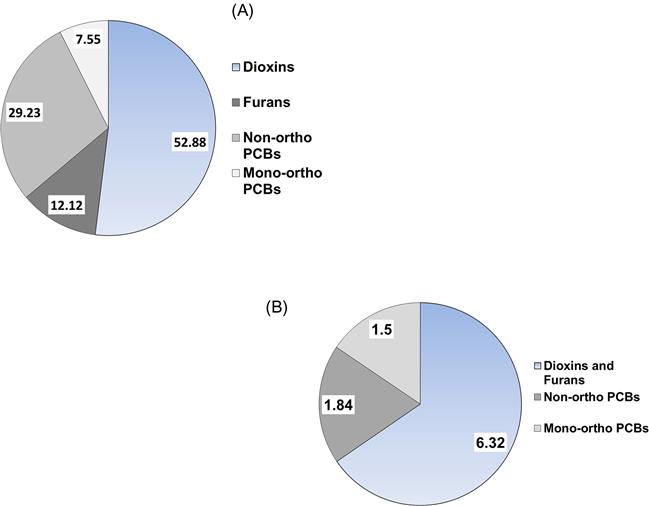
As briefly mentioned above, the presence of PCBs, dioxins, furans, and other chlorinated and brominated contaminants in human breast milk is a global concern (Porta et al., 2008). In a sample of first-time Irish mothers, Pratt et al. (2012) found that 65% of the total dioxin-like concentration was due to PCDDs and PCDFs (Fig. 6.12A) and that their share of the TEQ (9.66 ng/kg) was a proportional 62% (Fig. 6.12B). As one more example, in a sample of Japanese brown frogs (Rana japonica) mono-ortho PCBs accounted for 72% of the dioxin-like substances by concentration, but only 2% of the 5.05 ng/kg ww TEQ (Figs. 6.13A, and B).
Polybrominated Diphenyl Ethers and Polybrominated Biphenyls
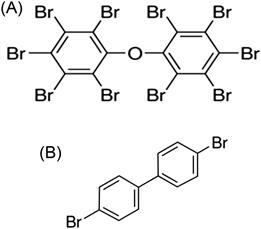
Instead of chlorine, bromine substitutes for hydrogen on the benzene rings of these chemicals. PBDEs and PBBs (Fig. 6.14) were used as flame retardants in a wide variety of industries including textiles, automotives, building materials, electronics, furnishings, aeronautics, and plastics. They share similar characteristics of persistence, bioconcentration and magnification, and lipophilicity with the other chemicals in this chapter. Both PBDEs and PBBs have 209 congeners because they have the same ring structure as PCBs. Commercially marketed PBDEs were sold as mixtures of several congeners. Penta- and octa-congeners of these brominated compounds are especially persistent and toxic.
PBBs were first voluntarily removed from the market by their manufacturer in the 1970s due to a major accident involving thousands of livestock. In early 1973, both PBB (sold under the trade name FireMaster®) and magnesium oxide (a cattle feed supplement sold under the trade name NutriMaster®) were produced at the same St Louis, Michigan plant by the Michigan Chemical Company. A shortage of preprinted paper bag containers led to 10–20 fifty pound bags of PBB accidentally being sent to the Michigan Farm Bureau Services in place of NutriMaster®. This accident was not recognized until long after the bags had been shipped to feed mills and used in the production of feed for dairy cattle. By the time the mistake was discovered in April 1974, PBB had entered the food chain through milk and other dairy products, beef products, and contaminated swine, sheep, chickens, and eggs. As a result of this incident, more than 500 contaminated Michigan farms were quarantined, and approximately 30,000 cattle, 4500 swine, 1500 sheep, and 1.5 million chickens were killed, along with more than 800 tons of animal feed, 18,000 pounds of cheese, 2500 pounds of butter, 5 million eggs, and 34,000 pounds of dried milk products (Michigan Dept. Public Health, 2011). While this may seem like ancient history, it is not beyond imagination that a similar accident could occur again with similar compounds. The manufacture of PBB was banned in the United States in 1976 after this catastrophe (US EPA, 2014).
In 2009, both PBDEs and PBBs were listed as POPs by the Stockholm Convention which has effectively reduced their production throughout much of the world. In the United States, bans on PBDEs are being applied on a state-by-state basis. Penta- and octaPBDEs, which are thought to be carcinogenic and are mobile in the environment, were the chief targets in these rules and banned from production in 2004. DecaPBDE was banned from production in 2010. As of 2015, 13 states had implemented regulations ranging from setting upper allowable limits of PBDEs in manufactured goods to total bans (NCSL, 2014). DecaPBDE has also been banned by the European Union. Manufacturing of PBDEs does not occur in Canada, but the use of penta- and oxyPBDEs and decaPBDEs are restricted. The purpose of PBBs and PBDEs—to provide protection against fire—is still of concern and other brominated and organophosphate compounds are being used instead.
Focus—In Situ Testing with Tree Swallows
Problem—Determine Whether the Contaminants in Wetlands Bioaccumulate and If They Have Any Effect on Wildlife Using Them
How would you go about addressing the problem described above? Undoubtedly there are a lot of ways, including doing expensive sampling of sediments, water, invertebrates, and wildlife near or in the wetlands. Unless you have your own analytical laboratory, extensive sampling is costly and the mere presence of contaminants, even in the wildlife species of interest, does not necessarily denote that they are affecting the animals. Organochlorines, for example, could be sequestered in fatty tissues where they have minimal consequences for healthy animals.
One way biologists have addressed this problem is to use avian species as sentinels. The choice of a sentinel species is complex. If you are testing the toxicity of a group of wetlands, the sentinel must extensively, preferably exclusively, use a specific wetland or small group of wetlands. It wouldn’t be desirable to have animals using a wide variety of ponds because you couldn’t be sure where the contaminants came from. Thus, the sentinel species should be rather sedentary. It might be highly desirable to sample the animals repeatedly throughout the course of a year and, if you could rely on at least some of the animals coming back in subsequent years, that might even be better. It would be ideal if the sentinel species provided some way for sampling without causing the death of the animal itself—to do minimally invasive procedures that would reduce stress and not hamper the survival of the animal or affect the population. You might also want to be reasonably sure of seeing some effects, at least in the wetlands you know to be most highly contaminated. The sentinel species should also have a widespread distribution so that similar studies can be conducted in many different localities.
There are some species that meet all of these criteria. Several researchers have studied freshwater turtles by collecting blood or sometimes euthanatizing them to collect organ samples (eg, Bishop et al., 1995). For the most part, turtles are sedentary and can be representative of a wetland. However, they also seem to be very hardy. While they bioaccumulate and even bioconcentrate some contaminants, there have been few studies that report adverse effects from these contaminants. Other species that may serve as sentinels include crocodilians and colonial species of birds. In these species, however, the investigator is pretty much restricted to studying where the animals are and has limited ability to study wetlands of his or her choice. Birds also migrate and contaminant residues obtained from their different residences may confound interpretations.
Another group of scientists have been using tree swallows (Tachycineta bicolor, Fig. 6.15) as sentinels. Tree swallow breeding distribution extends from central Alaska to central California and east to the Atlantic States, covering virtually all of Canada and the northern half of the United States. They readily use nesting boxes for breeding and can be attracted to them with relative ease. Tree swallows are almost exclusively insectivores and during the breeding season, the birds capture insects as they emerge from surface waters. They will usually feed within 500 m of their nest (Quinney and Ankney, 1985) and are opportunistic, so that if the wetland nearest their nest provides sufficient invertebrate food, they will feed extensively from that wetland. Tree swallows are gregarious and will nest in boxes that are closely spaced, which helps in ensuring an adequate statistical replication although this has been a problem in some studies. Finally, tree swallows lay four to seven eggs—this allows two eggs, the mass necessary to obtain reliable analyses, to be sacrificed for residue analysis while allowing the others to develop and hatch so that developmental morphology, hatching, and fledgling success can be compared to residue concentrations.

We mentioned that egg order is sometimes important when measuring contaminants—for some species the first laid eggs may have higher or lower concentrations than subsequent eggs in a clutch. Tree swallows show some of that depending on the specific contaminant (Custer et al., 2010). The authors did not find any difference in egg volume, weight, percent moisture, or percent lipids by laying order. Among the contaminants measured, PCB concentrations varied by as much as 60% from one egg to another within a clutch. The importance of egg sequence varied between years and even between clutches that were highly and lightly contaminated. Ultimately, it appears that the importance of egg sequence cannot be predicted ahead of time so the authors recommended randomly collecting two eggs per clutch and pooling their contents for analysis.
There have been many studies that used tree swallows in this type of experimental presentation and we cannot review them all. Instead, we will examine a few to provide enough information to develop an understanding of the process.
Pulp mills, factories that take trees and make them into paper, historically used many different chemicals in the process and contaminated many water bodies when they dumped their effluents into streams and rivers. In the 1980s and 1990s, many of these mills were either closed down or upgraded to reduce their levels of pollution. Tree swallows were used to evaluate the effects of these upgrades in British Columbia (Harris and Elliott, 2000). The investigators set up nesting boxes upstream and downstream of pulp mills along the Fraser and South Thompson Rivers in central Canada in 1993 and 1994. They found low concentrations of PCBs, PCDDs, and PCDFs. While there were no significant differences between upstream and downstream tissue concentrations, nestlings downstream on the Fraser River had the highest concentrations of organochlorines and those downstream on the south Thompson River had the highest TEQs. Nest success was lower downstream than upstream on both rivers but the authors found little direct evidence of specific contaminant effects. Downstream parents were less attentive than upstream birds, which might have been due to organochlorine toxicity in adults. Reduced attentiveness could also occur if there were fewer insects in downstream sites and this may or may not have been due to the contaminants. However, the authors could not make strong inferences on the effects of these contaminants on breeding success with only one year of study.
Also in Canada, Point Pelee National Park in Ontario has some hotspots of organochlorine contamination (Papp et al., 2005). Tree swallow nesting studies were used to determine that those birds nesting in the more PCB-contaminated sites had higher EROD activities than those that nested at less contaminated sites. However, reproductive success was not related to the level of contamination.
Synthesis of retinoids, including vitamin A, is disrupted by chlorinated hydrocarbon contaminants which can cause a series of problems related to vitamin A-mediated enzyme reactions. Corticosterone is a hormone whose elevated levels indicate stress. Using the tree swallow method, Martinovic et al. (2003) found that PCDD concentrations ranged from 5.4 pg/g to 79.5 pg/g in fledgling swallows along the St. Lawrence River in Canada and the United States. Various measures of retinoid correlated with PCDDs, causing the authors to conclude that PCDD was interfering with vitamin A pathways. Corticosterone was also elevated in highly contaminated sites, indicating that PCDD affected stress and the glucocorticoid axis that produces corticosterone. Both sublethal effects may result in numerous metabolic pathways that could compromise survival or reproduction.
The tree swallow egg removal procedure is flexible. For example, it has been used to compare physiological health among commercial apple orchards and reference sites (Bishop et al., 1998). In this case, pesticides such as pyrethroids and fungicides were the primary contaminants. Swallows in the orchards experienced slight anemia, somewhat impaired immune systems, and altered thymus and thyroid responses compared with birds from reference areas.
In addition to tree swallows, the egg removal for analysis accompanied by examination of the reproductive effects has been adapted to spotted sandpipers (Actitis macularia), belted kingfishers (Megaceryle alcyon, Custer et al., 2010), night herons (Nycticorax ncyticorax, Rattner et al., 2000), wood ducks (Aix sponsa, Augspurger et al., 2008), great blue herons (Ardea herodias, Baker and Sepulveda, 2009), and other species.
Study Questions
1. What halogens are most often associated with halogenated hydrocarbons?
2. Why are the number of congeners for PCBs, PBBs, and PBDEs limited to exactly 209? Do investigators have to look for all 209 PCB congeners when studying PCB contamination? Why or why not?
3. Of the molecular structures shown below, which is a PCB, PCDD, and PCDF?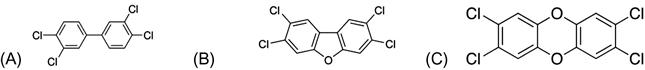
4. List the specific chemical formula names for the three molecules in question 3.
5. What factor on the PCB molecule determines if it is coplanar or not? Why does the molecular structure of coplanar PCBs make them function like dioxins?
6. Trace the changes in behavioral characteristics assumed by PCBs as the degree of chlorination increases.
7. Do dioxins occur naturally in the environment?
8. Most soil that is contaminated with PCBs or dioxins is physically removed and stored in landfills. Discuss some environmental problems associated with this type of disposal.
9. Why is TCDD the standard for toxicity comparison among dioxins and dioxin-like PCBs and furans?
10. Provide some reasons why male mammals tend to have higher PCB concentrations in their tissues than female mammals.
11. Explain how the P450 cytochromes can increase the toxicity of PCBs and related molecules.