1874
Tetrahedral Carbon Atoms
Jacobus Henricus van ’t Hoff (1852–1911), Joseph-Achille Le Bel (1847–1930)
The idea that chemical compounds have defined three-dimensional shapes caused by the arrangement of their bonded atoms is now such a foundation stone of chemistry that it’s hard to imagine a time when this property wasn’t known. In the second half of the nineteenth century, though, chemists were still thinking two-dimensionally, grappling with the basic structures of molecules and trying to understand what gave rise to chiral compounds.
Dutch chemist Jacobus Henricus van ’t Hoff attracted attention when he published a booklet in 1874 with his bold conjecture that single-bonded carbon atoms arrange their bonds in three dimensions, as if they were tiny tetrahedrons (triangular pyramids). French chemist Joseph-Achille Le Bel had independently come to the same conclusions in this same year, and for good reason. These ideas had a lot of explanatory power. For one thing, they gave an immediate reason for the origin of chirality. Van ’t Hoff took time in his paper to explain just how these principles applied to French chemist Louis Pasteur’s findings with the different forms of tartaric acid. Van ’t Hoff suggested that a tetrahedral carbon with four different groups attached to it could exist as two mirror-image isomers. His theory also reinforced the idea that molecules had characteristic shapes, a factor that became critical when explaining their properties.
For a theory that fit the observed facts so well, van ’t Hoff’s proposal attracted some fierce criticism. Part of this was due to the pamphlet’s unusually liberal use of illustrations, which in later versions even included cutouts for paper models of the tetrahedral carbons. The influential chemist Hermann Kolbe referred to van ’t Hoff’s work as “being dragged out by pseudo-scientists from the junk-room” and “devoid of any factual reality.” But van ’t Hoff was, in fact, the first winner of the Nobel Prize in Chemistry.
SEE ALSO Chirality (1848), Structural Formula (1861), Fischer and Sugars (1884), Asymmetric Induction (1894), Sigma and Pi Bonding (1931), The Nature of the Chemical Bond (1939), Conformational Analysis (1950), Resolution and Chiral Chromatography (1960), Enzyme Stereochemistry (1975)
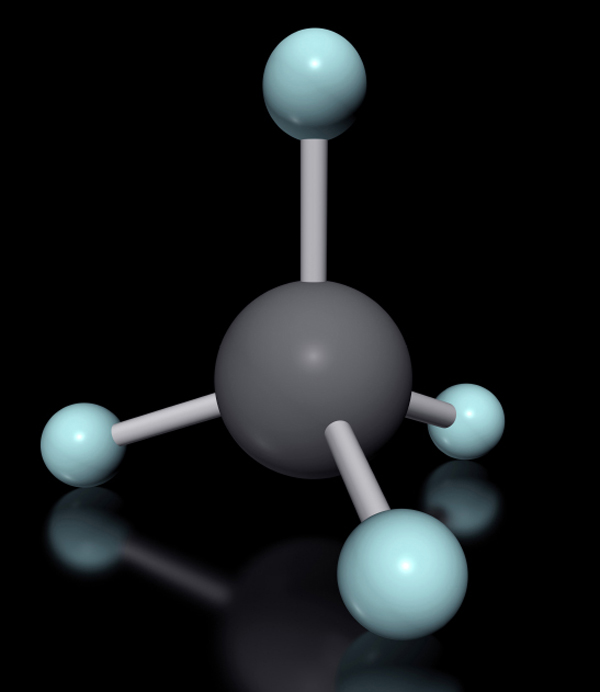
This illustration shows the basic tetrahedron that a four-bonded carbon makes. If you colored each outer ball a different color, you’d find that you could have two mirror-image versions that can’t be rotated to give the other one: a chiral carbon.