Learning Objectives
To understand when and how to use mammography, digital breast tomosynthesis, ultrasound, contrast-enhanced mammography, and magnetic resonance imaging for the diagnosis and staging of breast cancer.
To realize the limitations of each imaging modality.
To understand the information that can be obtained with each imaging modality and their complementary value in this context.
13.1 Mammography

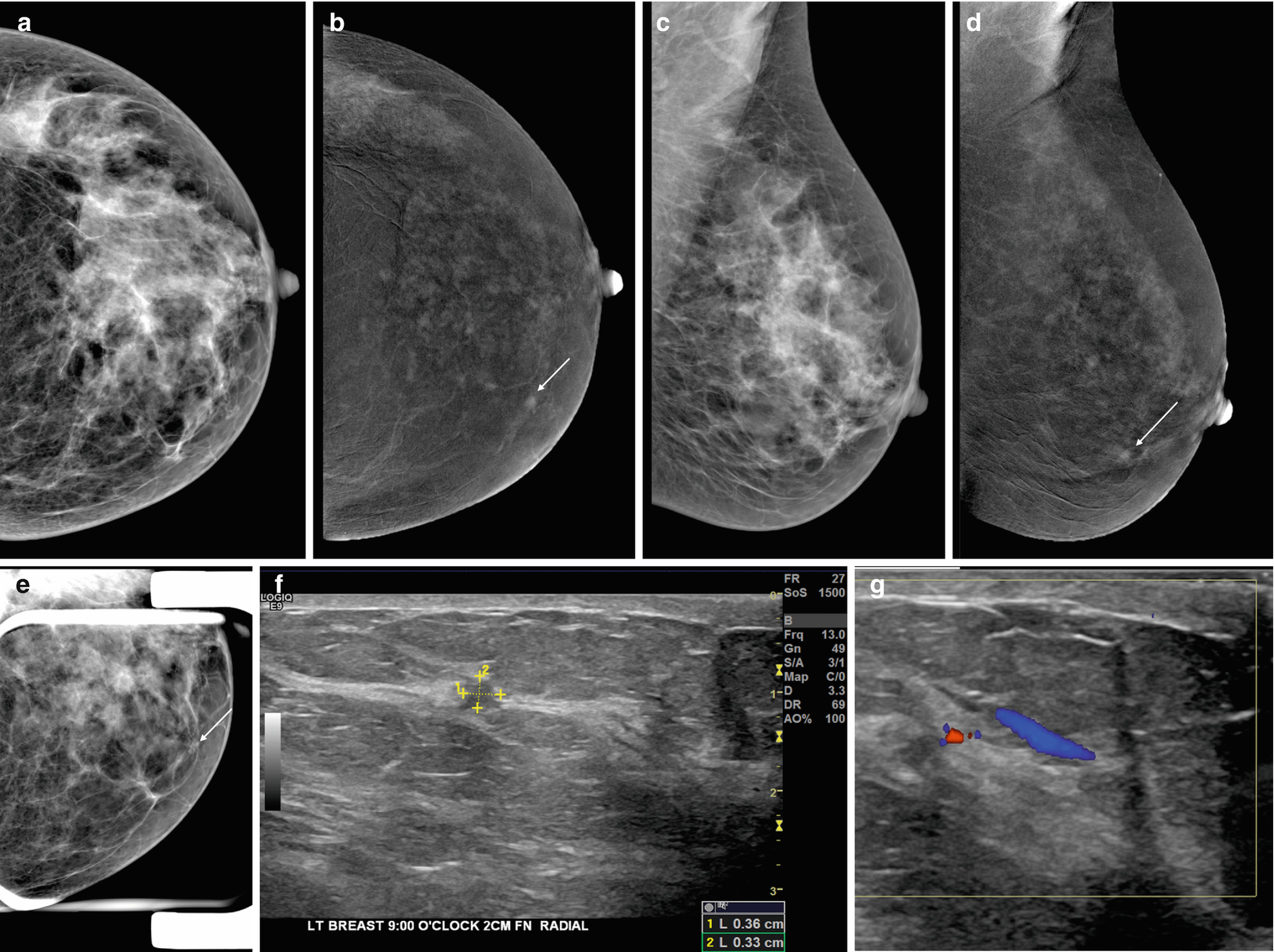
Screen-detected multifocal invasive ductal carcinoma III between the 9 and 10:00 axis of the left breast in a 72-year-old patient with a personal history of right breast cancer, breast conserving therapy and radiation treatment in 1983. (a, b) CC and MLO views: Post-surgical changes are present in the right breast. There are two new irregular shaped and partially spiculated masses in the 9 and 10:00 axis of the left breast posterior depth which are best appreciated on additional tomosynthesis views CC (c) and ML (d, spot). On targeted ultrasound (e) these correspond to two irregular shaped and marginated hypoechoic masses (9:00 6 cm from the nipple 0.6 × 0.5 cm, 10:00 6 cm from the nipple 0.4 × 0.4 cm)
Recommendations for breast cancer screening in average-risk women
UK National Health Service Breast Screening Programme | U.S. Preventive Services Task Force | American Cancer Society | National Comprehensive Cancer Network | American College of Radiology/Society of Breast Imaging | |
|---|---|---|---|---|---|
Clinical breast examination | Not recommended | Insufficient evidence to recommend for or against | Not recommended | Recommend every 1–3 years for women 25–39 years and annually for women 40 years and older | Not recommended |
Mammography initiation age | Offer starting at age 50 years | Recommend at age 50 years Age 40–49 years: decision to start screening mammography before age 50 years should be an individual one | Offer at ages 40–45 years Recommend at age 45 years | Recommend at age 40 | Recommend at age 40 |
Mammography screening interval | Three yearly | Biennial | Annual for women aged 40–54 years Biennial with the option to continue annual screening for women 55 years or older | Annual | Annual |
Mammography stop age | Continue until age 70 years Beyond age 70 years, women may continue to attend every 3 years | The current evidence is insufficient to assess the balance of benefits and harms of screening mammography women 75 years and older | When life expectancy is less than 10 years | When severe comorbidities limit life expectancy to 10 years or less | When life expectancy is less than 5–7 years |
13.1.1 Staging with Mammography



Multicentric invasive ductal carcinoma III and ductal carcinoma in situ high grade in a 54-year-old patient presenting with a palpable area of concern in the left breast for diagnostic mammography. CC and MLO views (a, b) and left magnification views CC (c) and ML (d). In the right breast there are no suspicious mass or tumor calcifications present. In the left breast there are pleomorphic microcalcifications spanning both the lower inner and outer quadrant. In addition, there is an enlarged axillary lymph node, which was confirmed to be metastatic
Key Point
Mammography is a two-dimensional image and relies on the identification of morphologic findings that are suspicious for breast cancer. Mammography is the mainstay of breast cancer screening and diagnosis.
13.2 Digital Breast Tomosynthesis
Digital breast tomosynthesis (DBT) images are created from repeated exposure of the breast tissue from different angles and data processing interpolated into multiple slices typically 0.5 mm thick through the breast tissue. Many retrospective and prospective studies have demonstrated that this technique is acceptable to women, increases the radiation dose by an average of 20%, and increases cancer detection by approximately 15–30% while reducing recall rates by 15–20% by decreasing overlapping shadows mimicking breast cancer [10]. While the technique is excellent for assessing soft tissue masses, architectural distortion, and asymmetries, the conspicuity and analysis of microcalcification were not improved [11]. However, recently, due to faster processing techniques manufacturers have been able to analyze all pixels instead of “binning” (combining pixels with the effect of reducing resolution) the data to reduce processing time. This means that fine calcification can now be more clearly identified with improved sensitivity and specificity.
DBT has been shown to be particularly useful in women with mixed to dense breast tissue (BIRADS B & C) but is not advantageous in women with very dense breast tissue. DBT is now increasingly used in the clinic either on its own with a 2D composite image or in conjunction with a standard 2D full-field digital mammography (FFDM) image. The advantage of using DBT is that the need for additional views such as the coned view or other supplemental techniques is no longer required [12]. In a recent meta-analysis, 17 studies were found where DBT was compared with 2D mammography in a screening setting. The pooled incremental cancer detection rate was 1.6 cancers/1000 screens compared with 2D FFDM with an overall absolute reduction in recall rates of 2.2%. However, there were differences between European and US-based studies with European studies showing a higher cancer detection rate of 2.4 cancers/1000 screens and a 0.5% increase in recall rates and US studies showing a reduction in the recall rates due the higher recall rates initially [13].
In the symptomatic setting, DBT has been found to have improved diagnostic accuracy compared with 2D mammography and improved reader confidence in distinguishing benign from malignant lesions and is more accurate in assessing tumor size and at identifying multifocal disease [14]. Techniques are also now available for image-guided biopsy using DBT to guide targeting.
Key Point
DBT has the potential to overcome the primary limitation of standard two-dimensional mammography, a masking effect due to overlapping fibroglandular breast tissue, improving diagnostic accuracy by differentiating benign and malignant features, and increasing lesion conspicuity, particularly in dense breasts.
13.3 Contrast-Enhanced Mammography
Contrast-enhanced mammography (CEM) is an emerging technology in breast imaging. CEM allows both a morphologic evaluation comparable to routine digital mammography and a simultaneous assessment of tumor neovascularity as an indicator of malignancy. Contrast-enhanced spectral mammography (CESM) acquires a low kV image and a high kV image simultaneously before and after the injection of iodinated contrast. Retrospective studies comparing CESM with standard 2D mammography show significant improvement in the sensitivity and specificity for detecting breast carcinomas with CESM; the sensitivity of CESM is 93–100% compared with 71.5–93% for mammography and increases the specificity from 42 to 87.7%. The patient populations in all these studies were either symptomatic patients or patients recalled to assessment after an abnormal screening mammogram [15, 16].
Invasive ductal carcinoma II in a 49-year-old patient who underwent contrast-enhanced mammography with a personal history of right breast cancer and mastectomy and reduction mammoplasty on the left. CC and MLO views (a, c), contrast-enhanced CC and MLO views (b, d), left CC spot compression view and diagnostic targeted ultrasound (f, g with color doppler). On mammography, left lower inner focal asymmetry that does not efface on spot compression correlates to a 0.4 cm enhancing mass on CEM. Targeted ultrasound shows an irregular shaped and marginated hypoechoic mass with vascularization
The disadvantage of this contrast examination is that approximately the same dose of iodinated contrast is injected intravenously, and sensitivity reactions can occur at the same rate as with computed tomography (CT) examinations. This means that CESM must be performed in a center with resuscitation facilities in place, and caution must be exercised in patients with impaired renal function, patients with allergies, and in the elderly.
The diagnostic accuracy in younger women and in those with dense breasts in the symptomatic setting is improved compared with 2D mammography [17].
13.3.1 Staging with CESM
A major advantage of CESM is that the ability to see additional foci of disease is enhanced hugely and, in many studies, it is comparable to MRI. Jochelson et al. found equal sensitivity between MRI and CESM for detecting the index cancer, although MRI was less sensitive for detecting additional tumor foci [18]. Lee-Felker et al. found that MRI had slightly higher sensitivity for the index lesion but equal sensitivity for detecting additional tumor foci [19]. Overall both studies showed that CESM had a significantly improved positive predictive value and specificity compared with MRI, as well as fewer false-positive interpretations. This means that once a cancer is suspected on imaging at the clinic visit, a CESM examination can be performed which has almost comparable sensitivity and specificity to staging breast MRI.
Key Point
CEM allows both a morphologic evaluation comparable to routine digital mammography and a simultaneous assessment of tumor neovascularity as an indicator of malignancy similar to MRI. CEM has an improved sensitivity and increases the specificity compared with mammography.
13.4 Ultrasound
Handheld ultrasound (US) has improved enormously over the last 20 years with markedly improved resolution and rapid image processing. While it is rarely used as a primary diagnostic tool, US is used in the majority of patients presenting with a clinical symptom as an adjunctive tool to further analyze a mammographic abnormality to determine whether a soft tissue mass is solid or cystic and to differentiate benign from malignant masses. It is also used when there is a negative mammographic examination, but the patient has a clinical symptom or palpable abnormality. The procedure is acceptable to patients, is safe with no ionizing radiation, but is operator dependent. The drawback for conventional US is that in breast tissue with extensive fibrocystic disease and shadowing, small tumors can be overlooked especially if they are invasive lobular disease. Ductal carcinoma in situ (DCIS) can be picked up now due to the improved resolution as microcalcification can produce a speckled pattern but DCIS with no calcification is difficult to detect.
Whole-breast US or Automated Breast US (ABUS) is a technique that is rapidly gaining acceptance. This technique requires the operator to undertake three positions with a flat panel US plate of each breast. The images are reconstructed to produce a 3D examination of the breast. This technique is showing promise in many clinical trials and may become the examination of choice for women with dense breasts in whom a supplemental examination is justified. A third of the United States, France, and Belgium have introduced supplemental imaging techniques such as US for women with BIRADS C & D breast density although in all cases this additional examination is insurance or self-funded. The literature supports the use of the supplemental imaging with studies reporting an additional 4 cancers/1000 screens when used with annual or 2-yearly screening. While the drawback for screening US has traditionally been that it had high recall rates ranging from 10 to 30%, a recent publication from Sweden has shown more promising results with ABUS with recall below 2.5% while good sensitivity is retained [20].
A most valuable aspect of US is the ability to rapidly undertake an image-guided biopsy. This can be done safely, in a timely manner at the first visit to the clinic and has a degree of accuracy without any precautions save checking for a bleeding diathesis.
13.4.1 Staging with US
US is widely used to confirm a diagnosis of cancer and to look for additional disease in the breast which is found in up to 20% cases. Additional disease is more often found toward the nipple and in the same quadrant as the index tumor.
Assessment of the axilla to look for abnormal lymph nodes is a very popular approach. The short axis diameter of axillary nodes is less than 5 mm in size, but in reality there is a large variation in normal lymph node size. Hence, the more reliable indicators of disease are abnormal shape (rounded), loss of echogenicity of the hilum, thickened cortex by more than 3 mm, or irregular lobulated cortex. When proving malignancy prior to surgery, US-guided core biopsies are undertaken.
US is also used extensively as a second-look tool in patients with abnormalities found on MRI particularly when the features are not diagnostic.
Lastly, US is used in localization techniques prior to surgery including the placing of a guide wire into the cancer to aid surgical procedure. This can be done accurately and efficiently under US guidance.
Key Point
US is widely used to confirm a diagnosis of cancer, to look for additional disease in the breast, for image-guided breast biopsy and localization, assessment of the axilla, and as a second-look tool in patients with abnormalities found on MRI.
13.5 Magnetic Resonance Imaging

0.4 cm invasive ductal carcinoma III medially in the right breast of a high-risk 51-year-old patient undergoing screening MRI. DCE-MRI (a, b, c) and MIP (d) shows round circumscribed mass with initial fast (b)/delayed plateau (c) enhancement signal intensity graph (e) sagittal view (f). Screening mammography and ultrasound were negative
13.5.1 Staging with MRI

MRI for staging of extent of disease in a 50-year-old patient with an invasive ductal carcinoma III with extensive intraductal component (EIC). On DCE-MRI (a, b), subtractions (d) and MIP (e). In the 9:00 axis mid depth there is a round irregular marginated mass with initial fast (b, f)/delayed plateau (c, f) enhancement measuring 1.4 × 1.4 × 1.1 cm with susceptibility artifact from clip marker. Extending from the index cancer into the anterior third of the breast there is a heterogeneous segmental non-mass enhancement representing the EIC. The index cancer and the contiguous non-mass enhancement span an area of approximately 3.8 × 1.6 × 1.5 cm. The non-mass enhancement engulfs a biopsy marker from a prior benign breast biopsy

Ductal carcinoma in situ (DCIS) intermediate grade, atypical ductal hyperplasia (ADH) and lobular carcinoma in situ classic type in 46-year-old patient with a history of right breast ADH and status post excisional biopsy undergoing screening MRI. Screening mammography and targeted second look sonography were negative. MRI-guided biopsy of the non-mass enhancement in the right breast retroareolar area shows right DCIS, ADH and LCIS. High resolution DCE-MRI (a–c), subtractions (e) and MIP (d) show in the early phase unique areas of non-mass enhancement with initial fast/delayed persistent enhancement (f)
DCE-MRI may also detect cancers that were occult on mammography and/or sonography in the contralateral breast in approximately 3% of women with unilateral cancer detected by mammography or US [38]. The detection of these initially unsuspected tumors may have a greater impact on patient outcomes than the detection of additional ipsilateral tumor foci as these would not be treated with concomitant radiation therapy. Although patient prognosis is determined by the size and grade of the index cancer, early detection of second cancers may be associated with a slight increase in survival, especially in patients younger than 50 years old [22]. Another indication of pretreatment breast MRI is as a problem-solving tool when tumor size differs significantly among imaging modalities or clinical examination and to evaluate eligibility for partial breast radiation therapy [21].
Key Point
DCE-MRI is the most sensitive modality for breast cancer detection with excellent sensitivity and good specificity. MRI is used for the assessment of disease extent and detection of additional lesions. DCE-MRI is more useful than mammography and US when staging multifocal and multicentric disease or when DCIS is present.
13.6 Concluding Remarks
In conclusion, imaging plays a pivotal role in breast cancer detection and staging and helps in guiding treatment decisions. Imaging modalities for diagnosis and staging of breast cancer comprise mammography, DBT, ultrasound, CEM, and MRI. Whereas mammography is the mainstay of breast cancer screening and diagnosis, other imaging modalities such as DBT and CEM have emerged with the potential to overcome limitations in sensitivity and specificity adding valuable information in breast cancer staging. US is widely used to confirm a breast cancer diagnosis, to look for additional disease and for image-guided breast biopsy and localization, staging of the axilla, and as a second-look tool in patients with suspicious findings on MRI. DCE-MRI remains the most sensitive modality for breast cancer detection with excellent sensitivity and good specificity and is more useful than mammography and US for the assessment of disease extent and detection of additional disease. Each imaging modality has its limitations and advantages and therefore may be used in conjunction to facilitate an optimal breast cancer staging and treatment.
Take-Home Messages
Mammography is the mainstay of breast cancer screening and diagnosis.
Breast US is widely used to confirm a diagnosis of breast cancer, to look for additional disease in the breast and for image-guided breast interventions.
DBT, CEM, and MRI increase cancer detection, especially in women with dense breasts at increased risk of cancer.
MRI of the breast is superior to other imaging modalities for the assessment of disease extent and detection of additional disease.

Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.