
The history of life is full of fast starts and odd experiments.

This planet did not start out as a cradle of life—in its earliest years, it was a hellscape. We wouldn’t recognize our world; a time-traveling visitor would need a space suit to survive for even a second. The atmosphere was a thin gruel of carbon dioxide (CO2) and nitrogen, entirely devoid of oxygen. The ground was streaked with lava, the sky sundered by volcanic lightning. Noxious chemicals bubbled to the surface and into the atmosphere: ammonia, sulfates, formaldehyde.1 The oceans grew, condensing out of the planet’s crust or falling from the sky as rain, but also being delivered piecemeal by incoming asteroids that bore frozen water.2 And complex chemicals were suspended dilutely in that ice from deep space, seeding the young planet with the molecular materials of life.3 It was in that alien, chemical stew that the very first elements of life appeared: nucleic acids and proteins. Just a few hundred million years after the crust cooled from magma, life had a lease on Earth.
That life would thrive, but not without crises, and not without experiments and failures and eventual successes. And in those early eons, the oceans swaddled the life of Earth, nurturing it and testing it and setting the conditions for life to persist. Eventually, the living tenants of the oceans grew abundant enough to change its chemistry, altering the very atmosphere of Earth, and building in the sea a complex web of species that exploded in diversity. Life took these skills onto land and transformed that realm as well. But while the cousins of mudskippers colonized the shores and eventually led to human beings, life continued to evolve into nearly every corner of the sea, finding food sources, then becoming food sources, and evolving the abilities to thrive in every kind of environment.
“Omne vivum ex viva,” Louis Pasteur once glibly proclaimed: life springs always from life.4 Intuitively it seems that the first life would be an exception, but it all depends on your definition of life. The very first self-replicating organic forms weren’t organisms per se; they were simply large molecules— molecular machines—and they probably began in the sea.5
The process was rapid. The first evidence of life—a signature shift in the isotopes of carbon found in rocks—appears 3.85 billion years ago in the Isua Supracrustal Belt of western Greenland,6 just 550 million years after the planet’s crust finally cooled from magma. Not only did life accrete quickly; it was also sufficiently robust to endure some serious punishment.
The early solar system was a new construction site, littered with asteroids left over from planet building. Careful cataloging of the Moon’s craters paints a picture of the rain of asteroids and comets onto our young planet. In those early eons, a series of cataclysmic asteroid strikes had enough power to vaporize the young planet’s oceans and sterilize its surface.7 New early-Earth models suggest that life might have survived these catastrophes, but only if it were already widespread when they occurred. A microbial community that spanned the globe could perhaps hide in deep ocean crevices, buffered from the devastation of planet-killing asteroid strikes and feeding off chemicals bleeding from the molten mantle. Once cellular life had sunk tendrils into ocean habitats as diverse as shallow pools and the deep sea, and once the early solar system was cleared of some of its original debris, life on Earth gained a permanent foothold.
Although life on Earth emerged fairly quickly, it took a long time to evolve past the basics. Recognizable living cells were present on Earth and common enough to form microscopic fossils 3.4 billion years ago.8 Rocks 3.4 billion years old in South Africa have a series of laminations and filaments suggestive of microbial mats formed in a shallow sea.9 Yet the world was still devoid of anything but microbes; for two billion years the only living things on our planet were single celled. Their sputtering metabolisms weren’t powerful enough to sustain anything grander. Life needed a new kind of metabolic engine to compete at the next level, and it was only invented in response to the planet’s first toxic waste crisis. That nasty poison was oxygen, loosed into the atmosphere by the worst of all primordial polluters: photosynthesizing microbes.10
The curves show the upper and lower estimates of oxygen in the atmosphere billions of years ago. Our modern atmosphere has an oxygen concentration (PO2) of 0.21 atmosphere (atm). Redrawn from Holland, H. D. 2006. “The oxygenation of the atmosphere and oceans.” Philosophical Transactions of the Royal Society B 361:903–915.
Photosynthesis uses sunlight to form sugars from CO2. The first forms of photosynthesis arose as early as 3.8 billion years ago and were thought to have been profoundly different from those common today. Most importantly, they did not yet produce oxygen.11 Oxygen is a home wrecker. The oxygen atom itself binds easily to other atoms, disrupting their chemical bonds. Oxygen atoms insinuate themselves slyly into nearly everything they encounter, breaking bonds faster than after a celebrity marriage. The word “oxygen” is itself derived from oxys: Greek for “acid.” And because of its disruptive chemical properties, oxygen destroys delicate RNA and DNA molecules, and even disrupts the more stable proteins of cellular life.
Single-celled microbes called cyanobacteria first started eating sunlight and producing oxygen more than two and a half billion years ago,12 dumping foul oxys into an early-Earth atmosphere that was likely nitrogen heavy.13 Various chemicals in the atmosphere and the soil absorbed that oxygen, “reducing” it and thereby protecting life’s fragile foothold. The balance held for a while, but as the cyanobacteria multiplied and oxygen production soared, something had to give. About a billion years ago, oxygen started to accumulate like junk in the garage.14
The Great Oxygenation Event was a catastrophe for life on early Earth. Only a few organisms were prepared to make use of the now-ubiquitous poison. But, as Jurassic Park’s Ian Malcolm declared, life finds a way. It found a way to feed on oxygen, using the crackling chemical energy of its bonds to power a new and mighty metabolic engine. If we think of metabolism without oxygen as a puttering outboard motor, then metabolism burning oxygen is a roaring Ferrari sports car.
Most of what we consider as “advanced” cell features, embodied in a line of cells called the eukaryotes, followed in the wake of this transition to an oxygen metabolism. These features importantly include subcellular organelles called mitochondria, which capture oxygen and burn it chemically to release its energy for the cells’ benefit. Mitochondria were once free-living bacteria with oxygen metabolisms:15 they were co-opted by the cells of our earliest ancestors, and they gifted these cells with the ability to burn oxygen too. Our existence as a species—indeed, the entire organization of life on Earth as we know it—is the unintended consequence of the use of oxygen after this toxic waste dumping.16
Long before the Great Oxygenation Event, the family tree of life on Earth had its trunk split in two. Of course, both bifurcations consisted of microbes— there was nothing else alive at the time. The first branch was composed of the cyanobacteria and other “normal” bacterial microbes. The second branch emerged around the same time, made up of microbes evolving to endure in stressful environments17 or living on a chemical diet without sunlight. They are Archaea—the extremophiles—the toughest creatures ever to live.18
They’re nothing much to look at: tiny oblong masses beneath an electron microscope. For a long time, we thought they were just bacteria. With the advent of gene sequencing, biologists noticed a huge genetic gulf between these extremophiles—found in salt lakes and deep-sea sulfur vents—compared to typical bacteria. In response, taxonomists created a name for this entirely new domain of life: Archaea.19 More and more of these creatures have been discovered at the margins of the world, living in places where little else could survive: the hot springs of Yellowstone National Park,20 the hydrothermal vents on the floor of the ocean, the oxygen-poor deep sea. As creatures of early Earth, Archaea tend to get crowded out by latter-day microbes. So they remain in extreme environments, in the closest analogues to the planet they lost.
Archaea can grow at temperatures exceeding 230° F (110° C), well above the boiling point of water. The Archaean Pyrolobus fumarii coats hydrothermal vents, 6,000–8,000 feet below the ocean surface, where sulfur and other toxic chemicals spew from Earth’s crust at temperatures of hundreds of degrees. These creatures hold the world record for growth at a high boil. They can survive an hour at autoclave temperatures of 250° F (121° C) and find temperatures of 203° F (95° C) too cold to reproduce well.21 No multicellular animal or plant can grow at such temperatures (see Chapter 8), and so the hottest places on our planet are solely tenanted by microbes. But microbes used to rule not just in high heat, but everywhere.
For a long moment in evolutionary history, across our entire planet, tiny microbes qualified as the most complex organisms on the planet. Eventually, some were able to form larger structures: thin layers of bacterial cells and secreted limestone piling one atop the other into mounds called stromatolites that persist three billion years later. These were still microbial constructions; no organism bigger than a single cell existed on Earth for eons.
Precisely when or how the jump was made from microbes to animals is not recorded. Fossil records are notoriously patchy, like the picture on an old television set. Static swells the further back you go. Large jelly-like organisms of many species all lumped together under the generalized name Ediacara appear in 575- to 542-million-year-old mud deposits.22 Other early cell clusters look like embryos of large creatures, though they might just be groups of single-celled protists.23 Tiny swimming discs like the bells of jellyfish wafted through the sea. On the floor were soft organisms that looked like disks, bags, toroids, or quilts.24 Whether these lines diversified into the life on modern Earth is ultimately unknown. They could be failures—snipped-off stubs on the evolutionary tree—or they could be the ancestors of all current animal life.
Our understanding of these early experiments in multicellular life completely changed in 1909 when paleontologist Charles Walcott walked into a quarry in British Columbia, Canada. He stood agog: before him spread an ancient deposit of ossified mud, more than 500 million years old and about the size of a city block. Dubbed the Burgess Shale, it remains to this day the planet’s best-preserved record of ancient marine organisms. This hunk of old ocean floor might be the most important discovery in modern pale-ontology.25 It documented, for the very first time, a worldwide biological revolution.
This enigmatic Ediacaran fossil represents one of the first multicellular species. But whether it is an animal, a fungus, a lichen, or something entirely unlike life today remains a mystery. Photograph by Meghunter99.
Cataloging a fossil dig like the Burgess takes a long time and an enormous amount of work. As Walcott, his family, and an army of paleontologists mined away and meticulously recorded their findings, based on more than 65,000 specimens, amazement with the newly discovered species grew. The creatures of the Burgess Shale were hard to fit into the normal taxonomy of living invertebrates. Their bodies were like unique jalopies assembled from random spare parts. Odd feeding trunks, long spiny legs, bony lobed fins, the wrong number of compound eyes—all these and more were trapped in the mud, slapped haphazardly onto animals that looked more like sci-fi cartoons than actual living creatures.
Wiwaxia was a small, scaled slug-like creature studded with petal-like flaps. Marrella resembled a brine shrimp wearing a motorcycle helmet, trailing long graceful tentacles from its mouths past its tail. Odaraia looked a bit like a fish in a hot dog bun, its torpedo-shaped body bulwarked on either side with large translucent carapace shells. Two compound eyes made of many facets, tiny feeding appendages near the mouth, and a bizarre three-finned tail rounded out Odaraia’s alien appearance.
The modern invertebrate world has long claimed its share of anatomical oddities, which biologists and taxonomists have labored hard to catalog. The Burgess Shale tossed a whole new menagerie on the table and threw taxonomy into a tailspin. All this new variety showed that the Cambrian was a unique period in natural history, when countless different forms leapt into existence in a geologic eyeblink: a worldwide phenomenon dubbed the Cambrian Explosion.26
The Burgess Shale is a snapshot taken about 505 million years ago. It captures the event in full bloom, the seas writhing with different body forms that all appeared in an extremely short period. Suddenly, without any obvious precipitating event, the sea had birthed advanced life at an evolutionary speed previously thought impossible. How could so many disparate creatures appear with no prior hint from the fossil record?
Darwin had died almost 30 years before Walcott walked into the Burgess Shale, but his evolutionary theories combined with twentieth-century science point to possible origins of the Cambrian Explosion. One satisfying theory, as popularized by the famous paleontologist Stephen Jay Gould, is called the empty barrel. The theory starts with the very first life: it was microbial, because it had to be. Low levels of atmospheric oxygen limited metabolism and created an environment hostile to large multicellular life forms. When at last there was enough oxygen to fuel powerful metabolisms, more complex creatures found themselves in much more benign environments, full of bacterial food and the metabolic energy needed to thrive.
Propelled by vast supplies of bacteria to feed on, and initially freed from competition’s cruel shackles in an ocean empty of animal life, evolution went berserk. Natural selection is supposed to weed out inefficiencies, one of Darwin’s ideas, but in the early Cambrian oceans any misbegotten jumble of genes and components capable of feeding itself would find success as a multicellular animal. There was almost nothing so absurd that it couldn’t survive somewhere. The oceans were an empty barrel, and life filled it with extreme speed and astonishing diversity.
Despite horrific extinction events in the intervening half-billion years, the marine world has never again been so empty. The Explosion’s products were here to stay, delicately twisting one another’s genomes through competition and predation. Ecology’s niches were always at least partially occupied; populations rose and fell but never completely vanished. As Andrew Knoll notes in Life on a Young Planet, “no one who has trekked through thick successions of Proterozoic shale or limestone can doubt that Cambrian events transformed the Earth. Cambrian . . . evolution may have taken 50 million years, but [that era] reshaped more than 3 billion years of biological history.”27
Besides the empty barrel, there is another plausible theory to help account for the Burgess Shale’s sudden diversity. It springs from new data from a very different kind of biological research: genetics. Advanced gene sequencing opened up new avenues of research and helped provide a different way of estimating when major taxonomic groups evolved.
When two groups diverge, their genes begin to accumulate different mutations. If we know the rate at which these mutations build up, and we know how many mutations separate the two groups, we can estimate how long ago they diverged. This mechanism, called the Molecular Clock, is a way to measure time through DNA, counting tiny mutations like ticks of the second hand and projecting eons across the evolutionary gap from starfish to lobsters.28 It tells us that the DNA of modern invertebrates is too different from one phylum to the next to have started accumulating mutations from the time of the Burgess Shale. Instead, the different types of invertebrate life must have diverged millions of years before—perhaps hundreds of millions.29
But if life has such a deep history, if arthropods and mollusks existed before the Cambrian, then why didn’t these different forms appear in the fossil record? Several possibilities might occur to you: (1) they were there all along, and produced fossils, but we have not found them yet; or (2) they were there but didn’t produce fossils, because they didn’t have body structures for fossilization.
Skeletons produce the best fossils. Soft tissues rot quickly, but hard parts—crab carapaces, snail shells, and fish bones—endure a long time. Suppose the ocean before the Cambrian was full of half-inch octopuses—would we ever know? It turns out that octopuses have hard beaks made of a protein similar to fingernails that can make good fossils, so we would know. But what about a half-inch snail without a shell, or worms burrowing in mud, or tiny starfish with soft fleshy arms? None of these would leave more than a trace of movement left behind in the sand.
In fact such trace fossils have been abundantly found, starting about 600 million years ago.30 Complex three-dimensional burrows of worms and scratch marks from the world’s first scrabbling appendages are etched into medallions of mud. And they predate the appearance of the complex skeletons of the Cambrian Explosion. So the explanation partly may be that many kinds of creatures existed before the Cambrian Explosion, but without fancy hard skeletons. Then what happened? Perhaps it was the planet’s first arms race.
As animals evolved into larger, mobile forms, they moved from grazing on helpless microbes to feeding on one another.31 Predators needed sharp claws and strong limbs to shred their prey. That prey, unwilling to be dinner, evolved defenses: shells, carapaces, spines, and toxins. In turn, predators countered with stronger jaws and teeth. And in response, prey evolved more defenses. Bit by bit and stage by stage, the animals may have evolved the weapons and the armor and the defenses that we see so abundantly in the fine fossils of the Burgess Shale. An explosive arms race that did not destroy life but diversified it.
The Burgess Shale is an antique fair of life, packing thousands of species into a modest mud parking lot. Over years of analysis, paleontologists have isolated a handful of creatures they thought most fundamental to life’s later story. Some were winners, and others were magnificent losers. But they all thrived for a time. Their disparate features illustrate just how unpredictable evolution can be when left to run wild.
In 1911, Walcott named a creature he thought of as a segmented worm, calling it Pikaia gracilens to convey the grace he imagined in its movement. About 2 inches long and delicately segmented, it was lumped together with about thirty other worm fossils that were later sent to a young Englishman named Simon Conway Morris. But Conway Morris, an early practitioner of three-dimensional fossil excavation techniques, quickly realized this was no worm. A hard central structure ran from one end to the other, girded on either side by zig-zagging bands of muscular tissue. Conway Morris was looking at a primitive backbone; a feature that would eventually grow into the crucial pillar of our nervous systems. The banded patterns in Pikaia were muscles bearing more resemblance to the repeated backbone structures of vertebrates than to anything ever seen in a simple worm.32 Precisely what Pikaia did in those seas of 500 million years ago remains a mystery, but it was fairly rare and disappeared not long afterward. Gould doubted that Pikaia was a universal vertebrate ancestor, but it seems to be our closest Cambrian relative. For Gould, Pikaia and its relatives were the thin line of life that perpetuated our kind from the heart of the Cambrian Explosion to the rest of evolution’s theater. This thin proto-spine in the Middle Cambrian suggests that the vertebrate body plan—so dominant today—was just one spinning spot of color in life’s early kaleidoscope.33
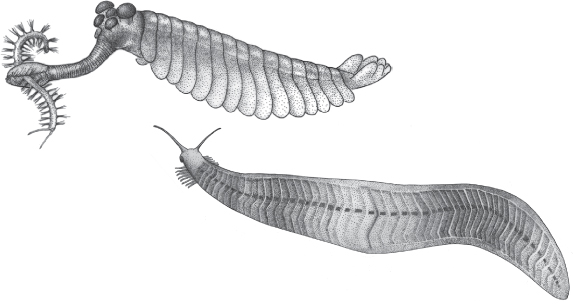
Opabinia regalis (above) and Pikaia gracilens (right), two enigmatic animals from the middle Cambrian Burgess Shale. From the Burke Museum: Opabinia regalis drawing by Mary Parish; Pikaia gracilens drawing by Laura Fry.
Compared to Pikaia, Opabinia regalis is an even bigger Burgess oddity. Though only a few inches long, the unveiling in 1972 of a new drawing of it to the Oxford Paleontological Association was met with incredulous laughter.34 Opabinia looked so bizarre that even the assembly of sober scientists could react no other way. The animal is oblong, a slightly engorged cigar trailing frond-like gills from the top of its carapace. A frondy tail propelled it slowly along the bottom, while five eyes (rare among arthropods, which tend to pair off their body parts) sat on stubby stalks. The gut ran tail-to-head in familiar fashion but took a bizarre U-turn at the head to form a backward-facing mouth. And protruding like a segmented vacuum hose from the head was a thin prehensile trunk. The nozzle-like device was tipped with spiny pincers, but it was also the perfect length to reach back to the mouth. Opabinia likely trolled the muddy bottoms of the Cambrian sea, rooting in the muck for tiny creatures.35
As in the case of Pikaia, early researchers tried to shoehorn Opabinia into a clean taxonomic bracket. Walcott considered it the perfect early arthropod!36 But later reconstructions by Oxford paleontologist Harry Whittington broke the shoehorn by showing the little creature could not fit into arthropodan preconceptions. Opabinia stubbornly resisted classification and essentially has been left out of the hierarchy of life.
Stephen Gould, among others, saw Opabinia as a stunning reinvention of the conventional: the key to understanding the entire Cambrian Explosion. Put simply, Opabinia resembles nothing in the modern world. Its features are so disparate, so far afield of what was expected in an arthropod, that it forced paleontologists to discard their assumptions about what was necessary for an organism to be successful. The residents of the Burgess Shale, unusual as they seem today, lived and fed and fought and died just as today’s creatures do. Which one was part of a line of animals that survived and thrived, and which one was in a group that dies out might have been preordained by their basic biological features. But maybe just as likely is that things didn’t have to unfold this way.
One of Gould’s largest contributions to popular evolutionary thought was his emphasis on the role of chance. Gould loved to talk about cathedrals and to use sports metaphors, and in Wonderful Life he keeps returning to the idea of “game tape”: the idea that natural history, were it to be replayed, might unfold very differently. Consider a famous football game: Super Bowl 44, played in February 2010, when the New Orleans Saints defeated the Indianapolis Colts. If you watch a replay of the game (a record massively more complete than any fossil bed), you can state with confidence that the Saints were the better team. This touchdown, that catch—you can point to every event that led to the natural result: 31-17, New Orleans. But of course, anyone could rightly object: “That was one game.” If the game were replayed, any number of things might be different.
Let’s examine one play from that game. Following The Who’s colorful half-time performance, the Saints attempted an onside kick to try to steal a possession. Onside kicks are typically desperation maneuvers, used when a team is losing with little time on the clock. They fail more often than not. This time should have been no different; the ball took an impish hop away from the Saints and directly into the waiting arms of a Colts player. Yet he allowed it to bounce off his facemask, losing control and ultimately ceding possession to the Saints. It was a huge play, swinging momentum and placing the Saints in control for the second half. But if those events were replayed a dozen times, how many times would the Saints have come up with the ball? If the Colts recovered with great field position, wouldn’t Peyton Manning have led them to an immediate touchdown? It’s impossible to say.
The Play of Life is enormously complicated. Success and failure hinge on a staggering number of different factors, the number and importance of which cannot be known in advance. For that reason, the outcome will always appear “natural” despite being (more or less) capricious. A meteor impact, an algal bloom, or a temporary El Niño climate swing might snuff an otherwise promising species out of existence. Pikaia might have perished and taken all of human history with it. Gould looked at the Burgess Shale’s incredible fossils and saw them for what they are: one small snapshot of an enormous game already in progress. The winners of the Cambrian Explosion evolved into the species on Earth today, while the losers retreated as stunted relics squashed in the mud of ancient stones. What if the winners and losers were reversed? Gould explains in his own words:
any replay of the tape would lead evolution down a pathway radically different from the road actually taken. But the consequent differences in outcome do not imply that evolution is senseless, and without meaningful pattern; the divergent route of the replay would be just as interpretable, just as explainable after the fact, as the actual road. . . . Each step proceeds for a cause, but no finale can be specified at the start, and none would ever occur a second time in the same way. . . . Alter any event, ever so slightly and without apparent importance at the time, and evolution cascades into a radically different channel.37
All the hundreds of thousands of crustacean species on modern Earth can be grouped into four major groups. They are as different as crabs and sea monkeys (Artemia, brine shrimp), though they share a set of common traits like paired legs and segmented muscles. In the Burgess Shale—a spot of land so small that it is just one mudslide the size of a city block in one tiny corner of an enormous planet—crustaceans have been classified into twenty-four different groups.38 Multicellular life poured down many channels, but then subsequent evolution took a few of the most successful anatomical designs and used them as the basis for everything that would follow.
The Explosion’s creative chaos was pure and open; then the process of extinction broke life’s broad river into the tributaries we see today. Opabinia was a casualty of this process, perhaps no less deserving of an evolutionary future than Pikaia. But the tape played out as it did, and we (the distant beneficiaries of evolution’s early winners) get to wonder at the losers in studies of ancient graves.39
Gould makes the point: “The sweep of anatomical variety reached a maximum right after the initial diversification of multi-cellular animals. … Compared with the Burgess seas, today’s oceans contain many more species based upon many fewer anatomical plans.”40
The winners didn’t necessarily boast the best long-term solutions: maybe they were just the best for a short, special spurt. But then the winnowing began: the initial explosion of genetic creativity was bled white by the eons of extinctions and environmental changes that followed. After the Cambrian Explosion populated the world with odd and complex organisms—with wild versions of experimental life—the slow grind of competition and natural selection killed most of them off. And the winners began to diversify into the myriad related life forms we see in the oceans today. So even though a huge number of different body plans exists now, filling all the various tiny niches in the world’s countless environments, they pale in comparison to early Cambrian fauna.
Combine the two Gouldian analogies of the empty barrel and the game tape. The empty barrel means that extreme life forms were simply tolerated in a world that had not yet filled with big, complex life. The game tape analogy reminds us that when exuberant life was evolving with little competition in an empty ocean, extreme creatures are as likely to succeed as any other. What appears on life’s stage can surprise us, and many things seem impossible until suddenly they stand before our eyes. We may call these forms unlikely, but that is just because we do not see them in the modern world. And it tells us that what we have come to find “likely” are the forms that we see, not the forms of life that necessarily must be.
Every creature we see today has some kind of root in the Cambrian Explosion. Life originated in the oceans, was sheltered by the sea through cataclysms, and only much later was anything sufficiently advanced to shuffle free of its fluid embrace. In the tens of million years of the Cambrian Explosion, nature and evolution amply populated the stage. The Cambrian Explosion produced the oceans first superstars, the trilobites that dominated the seas. It forged the different life forms that would take over in succession: the cephalopod mollusks that would become the ocean’s fiercest predators 400 million years ago, and even the first rough drafts of our own vertebrate body plan. The march of complex ocean life had begun.