GENERAL SCIENCE PRACTICE SET 2
Choose the best answer for each multiple-choice question. This question set has 16 practice questions, which is the number of General Science questions you will see if you take the CAT-ASVAB.
-
The Earth experiences seasonal patterns of weather because __________.
- its distance from the Sun varies at different times of the year
- solar wind activity varies
- its orientation to the Sun is tilted on its axis
- the Moon's position relative to the Earth varies
-
Body functions such as heart rate and digestion that take place without having to think about them are regulated by the __________.
- somatic nervous system
- cerebrum
- neurons
- autonomic nervous system
-
Which factor is most important in determining how atoms of an element will chemically react with other atoms?
- the number of electrons in the outer shell
- the number of neutrons in the nucleus
- the chemical symbol of the element
- the atomic mass
-
Our Sun is classified as what type of star?
- yellow dwarf
- white dwarf
- yellow giant
- white giant
-
Shale and sandstone are types of __________ rocks.
- igneous
- sedimentary
- metamorphic
- compound
-
A DNA (deoxyribonucleic acid) molecule is shaped like a __________.
- sphere
- double helix
- oblate spheroid
- triple helix
-
A truck was driven
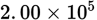 meters. At the beginning of the trip, the fuel tank contained 40,000 mL of fuel; at the end of the trip it still had 15,000 mL of fuel. What was the average fuel consumption, measured in liters/kilometer, for the trip?
meters. At the beginning of the trip, the fuel tank contained 40,000 mL of fuel; at the end of the trip it still had 15,000 mL of fuel. What was the average fuel consumption, measured in liters/kilometer, for the trip?- 0.0125 L/km
- 0.125 L/km
- 8.0 L/km
- 80 L/km
-
A net force of 15 N is applied to an object that starts at rest and accelerates to a velocity of 12 m/s in 4 seconds. What is the mass of the object?
- 3 kg
- 5 kg
- 12 kg
- 15 kg
-
A lunar eclipse is caused by __________.
- an anomaly in the Moon's orbit
- solar flares
- the Moon passing between the Earth and the Sun
- the Earth passing between the Sun and the Moon
-
Earthquakes occur due to movement of plates in the Earth's __________.
- asthenosphere
- lithosphere
- outer core
- lower mantle
-
Which of the following organisms has an exoskeleton?
- human being
- spider
- amoeba
- eagle
-
Our lungs convey __________ to blood cells and receive __________ and __________ from blood cells.
- oxygen, carbon dioxide, water
- oxygen, carbon dioxide, nitrogen
- carbon dioxide, oxygen, water
- nitrogen, carbon dioxide, oxygen
-
A fireplace is sealed behind a clear glass screen to prevent sparks and ashes from getting out. Nevertheless, a person sitting in front of the fireplace will be warmed by the heat of the fire primarily because some heat is still transferred by __________.
- convection
- radiation
- conduction
- osmosis
-
Sodium (Na), which is in Group 1 of the Periodic Table of the Elements, and chlorine (Cl), which is in Group 17, will bond together to form sodium chloride (NaCl). How will this compound behave when dissolved in pure water?
- The molecules will group together to form large crystals.
- The molecules will separate into Na and Cl atoms.
- The molecules will separate into negatively charged sodium ions and positively charged chlorine ions.
-
The molecules will separate into positively charged sodium ions and negatively charged chlorine ions.
-
Meteorology is the study of __________.
- meteors and meteorites
- weather
- the atmosphere and atmospheric phenomena
- the solar system
-
Human eggs are produced in the female's __________.
- uterus
- fallopian tubes
- cervix
- ovaries