Carsten Bolm

was born on March 8, 1960 in Braunschweig, Germany. He studied chemistry at the Technical University Braunschweig and at the University of Wisconsin, Madison, where he performed research under the supervision of H. Hopf and H.-J. Reich, respectively. In 1984 he received a M. Sc. degree in Madison and obtained a diploma in Brunswick. He then moved to the University of Marburg to start his doctoral work under the guidance of M. T. Reetz. After a postdoctoral stay in 1987/8 with K. B. Sharpless at the M.I.T. in Cambridge, Massachusetts, he worked in Basel with B. Giese to obtain his habili-tation in 1993. In the same year, he was appointed Professor of Organic Chemistry at the University of Marburg. Since 1996 he has held a Chair of Organic Chemistry at the RWTH Aachen. Carsten Bolm was Visiting Professor in Madison, Florence, Paris, and Milan. Among several awards, he received the Heinz Maier Leibnitz Award (1991), the ADUC-Prize (1992), the Otto Klung Award (1996), and the Otto Bayer Award (1998). He is a member of the advisory boards of Advanced Synthesis & Catalysis, New Journal of Chemistry, Synthesis, and Synlett. Furthermore, he published the book Transition Metals for Organic Synthesis (Wiley-VCH, 1998) together with M. Beller.
Scientific Sketch
The major focus of Bolm’s research is on asymmetric metal catalysis, synthesis with organometallic reagents, and pseudopeptides. Asymmetric synthesis has successfully been used for the preparation of enantiopure pharmaceuticals or agrochemicals. Within this area, catalytic approaches are considered most favorable. Catalysts of such type usually consist of a metal center and a ligand bearing the stereo-chemical information (Fig. 1), which ensures that the bond-forming process proceeds in a stereoselective manner.
Figure 1. Bolm’s ferrocene (S,Rp)-1
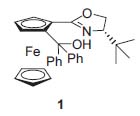
In this context one of the mojor research targets of Professor Bolm is to find developligands and metal complexes with multiple steregenic elements for the catalytic enantioselective addition of zinc reagents to aldehydes and imine derivatives (Fig. 2, J. Org. Chem. 1998, 63, 7860; Angew. Chem. Int. Ed. 2000, 39, 3465). In particular, aryl transfer reactions have been studied that lead to synthetically important diaryl methanols (3) and diaryl methyl amines with excellent enantiomeric excesses (Angew. Chem. Int. Ed. 2001, 40, 1488; Angew. Chem. Int. Ed. 2002, 41, 3692).
Figure 2. Asymmetric phenyl transfer onto substituted benzaldehydes in the presence of catalytic amounts of Bolm’s ferrocene (S,Rp)-1.
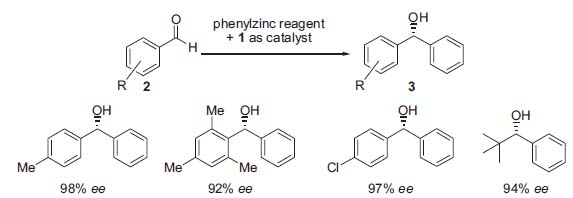
Figure 3. Basic pseudotripeptide structure.
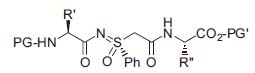
Another field of his work is focused on peptide mimetics. Due to their poor bioavailability and rapid enzymatic degradation, peptides have found only limited application as pharmaceu-ticals. Thus, Professor Bolm uses sulfoximines as chiral backbone-modifying elements to prepare new pseudopeptides with higher stability against enzymatic degradation. By this strategy, new enzyme inhibitors could result (Fig. 3; Chem. Eur.J. 2001, 7, 1118; Org. Lett. 2002, 4, 893).
Kaiserschmarren
From the King’s Hight in the Emperor’s City
Starting materials (serves 6):
Stewed fruits:
50 g raisins
10 mL (1 tbsp) rum
250 g sour cherries
170 g sugar
125 mL white wine
Dough
6 eggs
60 g sugar
pinch of salt
1 g lemon zest
12 mL (1 tbsp) double cream
65 g flour
butter
Soak raisins (50 g) overnight at room temperature in rum (10 mL) in a 50-mL screw cap vessel, which should occasionally be shaken. Next, fill sour cherries (250 g) into a 250-mL beaker and add sugar (60 g) and white wine (125 mL). Cover the reaction mixture and store cool overnight.
To synthesize the dough, whisk 6 eggs (weight class 2), sugar (60 g), fine lemon zest, and double cream (12 mL) with a dough mixer (KPG-mixer) at ca. 600 rpm in a 2-L porcelain bowl. Then add flour (65 g) and leave the dough to rise for 30 minutes. Heat butter (50 g) carefully in a frying pan and add some of the dough (CAUTION: squirting). Fry until golden underneath, then, using a fork, tear into small pieces (ca. 1 cm) and brown, turning frequently, and keep it warm in a muffle furnace.
Synthesis of stewed fruits: In a beaker, sugar is caramelized in molten butter (50 g). Using a Büchner funnel, filter off the sour cherries and raisins and then stir them into the caramel mixture. Quench the mixture with the filtrate (cherry juice/wine mixture). Pour the stewed fruits over the warm Kaiserschmarren, add some drops of orange liqueur, and dust the solid with icing sugar (3 g). Before serving, decorate with some lemon balm leafs.