Erick M. Carreira

was born in Havana, Cuba, in 1963. He earned a B.S. degree in 1984 from the University of Illinois at Urbana-Champaign under the supervision of S. E. Denmark and a Ph.D. degree in 1990 from Harvard University under the supervision of D. A. Evans. After carrying out postdoctoral work with Peter Dervan at the California Institute of Technology through late 1992, he joined the faculty at the same institution as an assistant professor of chemistry and subsequently was promoted to the rank of associate professor of chemistry in spring 1996 and full professor in spring 1997. Since September 1998, he has been full professor of Organic Chemistry at the ETH Zürich. He is the recipient of the American Chemical Society Award in Pure Chemistry, the Nobel Laureate Signature Award, the Fresenius Award, a David and Lucile Packard Foundation Fellowship in Science, the Alfred P. Sloan Fellowship, the Camille and Henry Dreyfus Teacher Scholar Award, the Merck Young Investigator Award, the Eli Lilly Young Investigator Award, the Pfizer Research Award, the National Science Foundation CAREER Award, the Arnold and Mabel Beckman Young Investigator Award, and a Camille and Henry Dreyfus New Faculty Award. He is also the recipient of the Associated Students of the California Institute of Technology Annual Award in Teaching and a Richard M. Badger Award in Teaching.
Scientific Sketch
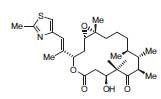
Carreira’s research program focuses on the asymmetric synthesis of biologically active, ste-reochemically complex natural products. Target molecules such as epothilone B (Fig. 1) are selected that pose unique challenges in a-symmetric bond construction (J. Am. Chem. Soc. 2001, 123,3611).
Figure 1. Epothilone B.
These asymmetric bond formations require new synthetic methodologies; another important field of research in Carreira’s group is the development of these methods. Drawing from the areas of organometallic chemistry, coordination chemistry, and molecular recognition, Carreira’s group is developing catalytic and stoichiometric reagents for asymmetric stereocontrol, including chiral Lewis acids.
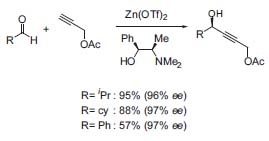
The asymmetric addition of terminal alkynes to aldehydes (Fig. 2) catalyzed by chiral zinc complexes is a useful tool for the preparation of chiral building blocks in natural product synthesis (J. Am. Chem. Soc. 2000, 122, 1806).
Figure 2. Asymmetric addition of alkynes to aldehydes.
Another powerful reaction is the transition metal-catalyzed aldol addition of silyl enol ethers, silyl ketene acetals, and dienolates to aldehydes. Catalysts for these transformations have been developed in Carreira’s group (Fig. 3).
Figure 3. Asymmetric addition of ketene acetals to aldehydes.
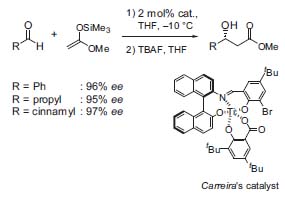
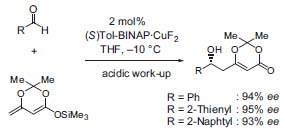
Starting with Ti(IV)-complexes (Carreira’s catalyst, Fig. 3, J. Am.| Chem. Soc. 1994, 116, 8837), new copper catalysts have been synthesized recently that are highly reactive and selective (Fig. 4,J.Am. Chem. Soc. 1998, 120, 837). O
Figure 4. Asymmetric dienolate additions.
Black Bean Soup
Starting materials:
450 g dried black beans
1 bay leaf
2 medium-sized green bell peppers, seeded and cut into small pieces
175 mL olive oil
4 cloves garlic, finely chopped
2 large onions
3 tsp ground cumin
1 tbsp oregano
2 tbsp cider vinegar
2 beef bouillon cubes
The black beans should be washed with cold water, and any beans that float to the top or are discolored should be removed and disposed of. The beans should be soaked overnight in cold water to cover 4 cm. The next day the water should be thrown away and the beans washed again with cold water. The beans are now ready for cooking. After covering with water by 5 cm, the bay leaf is added before bringing the beans to a boil over high heat. The heat can be reduced to low and the beans cooked until they are tender and almost cracked open, 2-3 hours depending on the quality and freshness of the beans. If a pressure cooker is used, this requires only 15 minutes of cooking at full pressure. The beans should be checked during the cooking, and more water can be added as needed.
As the cooking of the beans nears completion, the sofrito can be prepared. In a frying pan the olive oil is heated over low heat and then the garlic, onion, and bell pepper are added. This mixture is cooked with stirring until the onion is semi-transparent, approximately 10 minutes. The cumin and oregano are subsequently added and thoroughly mixed in.
The sofrito is added to the beans along with the beef bouillon cubes and vinegar. The mixture is thoroughly mixed and cooked over low heat covered for an additional 30-40 minutes until the beans crack open. Prior to serving, salt can be added to taste. The black bean soup can be eaten as a soup or alternatively over white rice.
«I was born in Cuba;
thus this recipe is a family one.»
Erick M. Carreira