Dieter Enders

was born 1946 in Butzbach, Germany. He obtained his diploma in 1972 and a Ph.D. degree in 1974 from the University of Giessen, Germany, under the guidance of D. Seebach. After a postdoctoral year at Harvard University with E. J. Corey, he returned to the University of Giessen, Germany, for his habilitation, where he was appointed lecturer in 1979. In 1980 he became associate professor at the University of Bonn, and since 1985 he has been full professor and director at the Organic Chemistry Department at the RWTH Aachen, Germany. Among his several fellowships and awards are the Heisenberg Fellowship of the Deutsche Forschungsgemeinschaft (1979-1980), the Leibniz Award of the Deutsche Forschungsgemeinschaft (1993), the Yamada Award of Japan (1995), the Max Planck Forschungspreis for Chemistry (2000), and the Emil Fischer Medal of the GDCh (2002). He is Editor in Chief of Synthesis and a member of the advisory boards of Synlett and Tetrahedron: Asymmetry.
Scientific Sketch
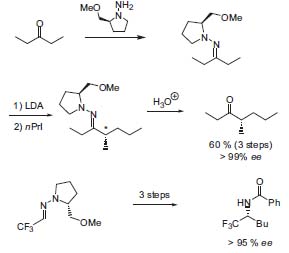
The current research interests of Dieter En-ders and his coworkers are focused on the development of highly stereoselective bond construction methods, their application in the synthesis of natural products and bioactive compounds in general, and combinatorial chemistry. A number of years ago, Enders developed a general methodology for asymmetric electrophilic substitutions of aldehydes and ketones masked as chiral hydrazones. As derivatives of proline, a chiral-pool compound, these so-called “Enders-auxiliaries” are easy to prepare. Using this method, α-substituted ketones or amines for instance are readily available by 1,2-addition (Fig. 1, Angew. Chem. Int. Ed. 1979, 18, 397; Org. Lett. 2001, 3, 1575; Tetrahedron 2002, 58, 2253).
Figure 1. Asymmetric syntheses with SAMP/ RAMP-hydrazones.
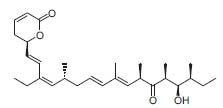
In many total syntheses of natural products, this asymmetric methodology has been used, e.g., (-)-callystatin A has been synthesized in this research group (Fig. 2, Chem. Eur.J. 2002, 8, 4272).
Figure 2. (–)-Callystatin A.
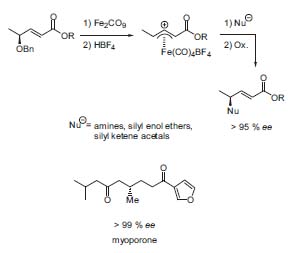
Another research topic is the iron-mediated al-lylic substitution. Chiral allylic ethers are transformed into their iron complexes: these complexes can be alkylated with several nucleo-philes. This method has also been used in some total syntheses, e.g., myoporone (Fig. 3, Synlett 1997, 421).
Figure 3. Iron-mediated allylic substitution.
Enders is also engaged in asymmetric catalysis employing nucleophilic carbenes. Further current projects involve metalated aminonitriles, silylated ketones, lactams, sulfonamides, and sulfonates.
Chicken à la Maritje
Starting materials (serves 4):
3 tbsp oil
1 onion
100 g mushrooms
500 g chicken filets
125 mL soy sauce or ketjap Manis
250 mL water
125 mL chopped tomatoes or tomato sauce
1 tsp Sambal Oelek
2 tsp brown sugar
1 tbsp balsamic vinegar 1 tsp starch
Heat the oil in a large pan or skillet. Chop onions, slice mushrooms, and cut chicken into serving pieces. Sauté onions, and add the mushrooms and chicken pieces. Fry them until golden brown on all sides. Add remaining ingredients and simmer, stirring, 10 minutes. Stir in the starch dissolved in water or ketchup, reduce the heat, and simmer stirring constantly until thickened.
Serve with rice and a crisp green salad.
«Maritje is the wife of a Dutch mathematician who was a post-doc at Harvard University in Cambridge, Massachusetts, at the same time as myself. We got the recipe from her: we love it - so do my kids.»
Dieter Enders