Burchard Franck

was born in Hamburg, Germany, in 1926. He graduated from Hamburg University with a diploma thesis on digitalis glycosides. Franck obtained his Ph.D. in organic chemistry in 1952 at the University of Göttingen, where he studied streptomyces antibiotics under the guidance of H. Brockmann. After his habilitation in 1959 in Göttingen, he accepted a chair at the University of Kiel in 1963. Six years later, he moved to Münster to become a full professor and director of the department of organic chemistry. He was visiting professor at the Massachusetts Institute of Technology, the University of Connecticut, Oslo University, as well as in Lausanne, Genf, and Fribourg in Switzerland; Guangzhum, Shanghai, and Beijing in China; and in India.
Among his numerous awards, he received the Medal of the Medical Society, Sendai, Japan (1971), the Richard Kuhn Medal of the Gesellschaft Deutscher Chemiker (1980), and the Adolf Windaus Medal of the University of Göttingen (1981). He was a member of the editorial board of Angewandte Chemie, Liebigs Annalen der Chemie, and Heterocycles.
Scientific Sketch
Burchard Franck (emeritus) dedicated his scientific career to natural product and bioorganic chemistry. Thus, his research group investigated the biosynthesis of porphyrins and developed the synthesis of novel porphyrinoids and macrocyclic aromatic compounds. Recently, he used porphyrins as building blocks for new aromatic 30-π-electron systems (Fig. 1, Angew. Chem. Int. Ed. 1997, 36, 221 3), an analogue of octaethylporphyrine, the most widely used porphyrine in the chemical and medicinal fields.
Figure 1. Synthesis of a superaromatic 30-π-electron porphyrine.
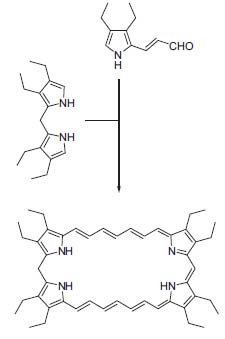
Franck’s research includes the photomedical application of porphyrines and other photosensitizers as well as the isolation, synthesis, and biosynthesis of biologically active compounds.
Due to the interest of biosynthesis of biologically active compounds, his group reported the stereoselective synthesis of tetradeuterated 1,24-dihydroxy squalene 2,3;22,23-dioxides by a double Sharpless epoxidation (Fig. 2, Tetrahedron Lett. 1997, 38, 383).
Figure 2. Synthesis of precursors for the enzymatic cyclization to triterpenes.
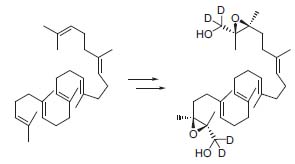
These precursors can be transformed under enzymatic conditions into tetracyclic triterpenes, which deserve attention as possible steroid biosynthesis inhibitors.
Labskaus
Starting materials (serves 4–6):
1 kg salt meat
4 young salted hering filets
500 g onions
6 pickles
4 soused herings
500 g red beet
1 kg potatoes
20 glard
I bay leaf
peppercorns
I fried egg per person
I rollmop per person
Boil the salt meat covered with water together with bay leaf and peppercorns for approx. 1.5 hours in a closed pot so that the meat becomes slightly soft. Boil the potatoes, remove the skin afterwards, and crush them while they are still hot. Chop the onions and stew them in the hot lard until glassy, then mix them with the finely chopped or minced salt meat and the crushed potatoes. Cut the hering filets into small strips and the pickles into small cubes. Combine them with the rest. Add some meat broth if necessary. Mix the chopped red beet without its juice with the labskaus. Fill the mixture onto a preheated dish and cover with a fried egg for each person on top. Arrange the rollmops separately.
Instead of serving salt meat, one can serve corned beef, which has to be chopped and stewed together with the onions.
Labskaus
«is a typical sailor’s meal, established at a time, when storage and freshness capabilities were limited on board and the smutje had the problem of preparing good-tasting meals from well-known residues. This is the reason that sharp tongues claim that sailors will retrieve everything they lost within the last year in their labskaus.»
Burchard Franck