John F. Hartwig

was born August 7, 1964 near Chicago, Illinois, and was raised in upstate New York. He received a B.A. degree in 1986 from Princeton University and a Ph.D. degree in 1990 from the University of California, Berkeley, under the collaborative direction of Robert Bergman and Richard Andersen. After an American Cancer Society postdoctoral fellowship with Stephen Lippard at the Massachusetts Institute of Technology (1990–1992), he joined Yale University in 1992, where he is now professor of chemistry.
Professor Hartwig is the recipient of the Dreyfus Foundation New Faculty Award (1992), the DuPont Young Professor Award (1993), the National Science Foundation Young Investigator Award (1994), the Union Carbide Innovative Recognition Award (1995 and 1996), an Eli Lilly Grantee (1997), an Alfred P. Sloan Research Fellow (1996–1998), the Camille Dreyfus Teacher-Scholar Award (1997), and the Arthur C. Cope Scholar Award (1998).
Scientific Sketch
Professor Hartwig’s research focuses on the discovery and understanding of new reactions catalyzed by transition metal complexes. The approaches are interdisciplinary between inorganic and organic chemistry, developing new metal chemistry and its application to synthesis as well as their mechanistic studies.
Traditionally, organometallic chemistry focused predominantly on forming and cleaving carboncarbon and carbon-hydrogen bonds. Meanwhile, the transition metal chemistry of amines, alcohols, ethers, and boranes has grown rapidly, providing major advances in the field. This will define new areas at the crossroads of inorganic, organometallic, and organic chemistry.
In that context, Hartwig’s group has developed, for example, a regiospecific rhodium-catalyzed functionalization of alkanes and polyolefins (J. Am. Chem. Soc. 2002, 124, 1164), a mild iridium-catalyzed borylation of arenes (J. Am. Chem. Soc. 2002, 124, 390), and a method for the formation of arylamines and aryl ethers from aryl halides or sulfonates (J. Am. Chem. Soc. 2001, 123, 12905).
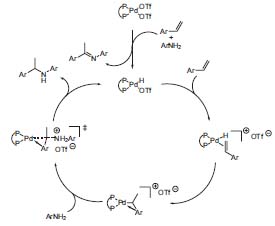
Another goal of Hartwig’s research group is the hydroamination of olefins using transition metal catalysis. Recently, he discovered the use-fullness of palladium-catalysts for the addition of aromatic and aliphatic amines to dienes and for the addition of aromatic amines to vinylarenes, with good enantioselectivity. The mechanism of the palladium-catalyzed addition of anilines to vinylarenes (Fig. 1) was revealed through the isolation of catalytic intermediates (J. Am. Chem. Soc. 2002, 124, 1 166).
Figure 1. Catalytic cycle of the hydroamination of vinylarenes catalyzed by palladium-diphosphine complexes.
Moreover, Hartwig developed a simple method to convert aryl halides and ketones, malonates, and other related carbonyl compounds to α-aryl carbonyl compounds in the presence of a base and a palladium catalyst. Familiar compounds that can be generated from these products include ibuprofen, naproxin, and tamoxifen. The reaction proceeds well and in many cases with low catalyst loadings. As part of their studies to understand this process, Hartwig and coworkers have generated both O-bound and C-bound palladium enolate complexes. These complexes undergo reductive elimination of the α-aryl ketone, ester, or amide product in good yields (J. Am. Chem. Soc. 2001, 123, 5816; ibid. 2001, 123, 8410).
Sorrel Soup
Starting materials (serves 4):
3 leeks (better) or onions or a mixture of both
4 tbsp butter
170 g sorrel (washed with large parts of the stem removed)
1 large boiled potato or 2 smaller ones (peeled if not blending with a food mill)
1.2 L chicken broth
salt and white pepper to taste
In a pot large enough to hold 2 L, sauté the leeks or onions in butter over medium heat for about 5 min. Cut the potato into four (or two if smaller) pieces and add to the pot. Add the sorrel and sauté for a few minutes until the sorrel has turned dark and wilted. Cover with the chicken broth (I made this once with water and it was not nearly as good; it’s better with homemade broth) and cook for about 45 min until the potatoes are soft. With a food mill or food processor blend until smooth.
Variant of Niçoise Salad
Inspired by the 2000 IASOC meeting
Starting materials (serves 4):
1/2 can Italian (darker) tuna in olive oil
50 g green and calamata olives
1 tbsp capers
2 small shallots
1 red pepper, roasted
1/2-1 chopped fresh tomato, if in season
juice from half a lemon and some zest
250 g pasta
Their version did not have any potatoes or egg, which I see in most Niçoise Salad recipes. I guess this isn’t really a Niçoise Salad, but a pasta with related ingredients. I also had a similar pasta on vacation in Venice.
Simply cook 250 g radiatore or other-shaped pasta to directions. Roast the red pepper on a gas stove or under the broiler until mostly black and, after allowing to cool in a paper bag, remove the skin without rinsing with water. Pit the olives. Julia Child says you need to mix each ingredient individually with olive oil and then combine them, but I mixed the ingredients together in a bowl, breaking up the tuna, adjusted the amounts to my taste, and added a generous amount of olive oil. Serve at room temperature.
«Although conference food is usually mediocre, I thought I would include two simple recipes that were inspired by excellent dishes at chemistry conferences I’ve attended. The first is a soup I made after attending the Bürgenstock conference in 2001 and the second, a pasta I started making after speaking at the Ischia Advanced School of Organic Chemistry.»
John F. Hartwig